A Multifunctional Reactor with Dry-Stored Reagents for Enzymatic Amplification of Nucleic Acids
- PMID: 29226671
- PMCID: PMC6310013
- DOI: 10.1021/acs.analchem.7b03834
A Multifunctional Reactor with Dry-Stored Reagents for Enzymatic Amplification of Nucleic Acids
Abstract
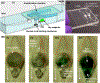
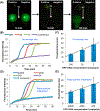
To enable inexpensive molecular detection at the point-of-care and at home with minimal or no instrumentation, it is necessary to streamline unit operations and store reagents refrigeration-free. To address this need, a multifunctional enzymatic amplification reactor that combines solid-phase nucleic acid extraction, concentration, and purification; refrigeration-free storage of reagents with just-in-time release; and enzymatic amplification is designed, prototyped, and tested. A nucleic acid isolation membrane is placed at the reactor's inlet, and paraffin-encapsulated reagents are prestored within the reactor. When a sample mixed with chaotropic agents is filtered through the nucleic acid isolation membrane, the membrane binds nucleic acids from the sample. Importantly, the sample volume is decoupled from the reaction volume, enabling the use of relatively large sample volumes for high sensitivity. When the amplification reactor's temperature increases to its operating level, the paraffin encapsulating the reagents melts and moves out of the way. The reagents are hydrated, just-in-time, and the polymerase reaction proceeds. The amplification process can be monitored, in real-time. We demonstrate our reactors' ability to amplify both DNA and RNA targets using polymerase with both reverse-transcriptase and strand displacement activities to obtain sensitivities on-par with benchtop equipment and a shelf life exceeding 6 months.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
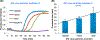

Similar articles
-
An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids.Biomed Microdevices. 2010 Aug;12(4):705-19. doi: 10.1007/s10544-010-9423-4. Biomed Microdevices. 2010. PMID: 20401537 Free PMC article.
-
A PCR reactor with an integrated alumina membrane for nucleic acid isolation.Analyst. 2010 Sep;135(9):2408-14. doi: 10.1039/c0an00288g. Epub 2010 Jul 9. Analyst. 2010. PMID: 20617276 Free PMC article.
-
Microfluidic devices for nucleic acid (NA) isolation, isothermal NA amplification, and real-time detection.Methods Mol Biol. 2015;1256:15-40. doi: 10.1007/978-1-4939-2172-0_2. Methods Mol Biol. 2015. PMID: 25626529 Free PMC article.
-
Simple Approaches to Minimally-Instrumented, Microfluidic-Based Point-of-Care Nucleic Acid Amplification Tests.Biosensors (Basel). 2018 Feb 26;8(1):17. doi: 10.3390/bios8010017. Biosensors (Basel). 2018. PMID: 29495424 Free PMC article. Review.
-
Emerging ultrafast nucleic acid amplification technologies for next-generation molecular diagnostics.Biosens Bioelectron. 2019 Sep 15;141:111448. doi: 10.1016/j.bios.2019.111448. Epub 2019 Jun 18. Biosens Bioelectron. 2019. PMID: 31252258 Review.
Cited by
-
Detection of Streptococcus equi subsp. equi in guttural pouch lavage samples using a loop-mediated isothermal nucleic acid amplification microfluidic device.J Vet Intern Med. 2021 May;35(3):1597-1603. doi: 10.1111/jvim.16105. Epub 2021 Mar 17. J Vet Intern Med. 2021. PMID: 33728675 Free PMC article.
-
A closed-tube, single-step, real time, reverse transcription-loop-mediated isothermal amplification assay for infectious bronchitis virus detection in chickens.J Virol Methods. 2020 Oct;284:113940. doi: 10.1016/j.jviromet.2020.113940. Epub 2020 Jul 17. J Virol Methods. 2020. PMID: 32687868 Free PMC article.
-
Microfluidics for the biological analysis of atmospheric ice-nucleating particles: Perspectives and challenges.Biomicrofluidics. 2025 Feb 27;19(1):011502. doi: 10.1063/5.0236911. eCollection 2025 Jan. Biomicrofluidics. 2025. PMID: 40041008 Free PMC article. Review.
-
Bridging the gap between development of point-of-care nucleic acid testing and patient care for sexually transmitted infections.Lab Chip. 2022 Feb 1;22(3):476-511. doi: 10.1039/d1lc00665g. Lab Chip. 2022. PMID: 35048928 Free PMC article. Review.
-
Sample-to-Answer Droplet Magnetofluidic Platform for Point-of-Care Hepatitis C Viral Load Quantitation.Sci Rep. 2018 Jun 28;8(1):9793. doi: 10.1038/s41598-018-28124-3. Sci Rep. 2018. PMID: 29955160 Free PMC article.
References
-
- World Health Organization (WHO). WHO Recommendations on the Diagnosis of HIV Infection in Infants and Children; WHO: Geneva, Switzerland, 2010. - PubMed
-
- Steinhagen K; Probst C; Radzimski C; Schmidt-Chanasit J; Emmerich P; van Esbroeck M; Schinkel J; Grobusch MP; Goorhuis A; Warnecke JM; Lattwein E; Komorowski L; Deerberg A; Saschenbrecker S; Stocker W; Schlumberger W Euro. Surveill 2016, 21, DOI: 10.2807/1560-7917.ES.2016.21.50.30426 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

