Group A Streptococcus encounters with host macrophages
- PMID: 29226710
- PMCID: PMC5771463
- DOI: 10.2217/fmb-2017-0142
Group A Streptococcus encounters with host macrophages
Abstract
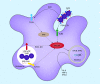
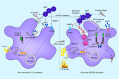
Group A Streptococcus (GAS) is a leading human bacterial pathogen with diverse clinical manifestations. Macrophages constitute a critical first line of host defense against GAS infection, using numerous surface and intracellular receptors such as Toll-like receptors and inflammasomes for pathogen recognition and activation of inflammatory signaling pathways. Depending on the intensity of the GAS infection, activation of these signaling cascades may provide a beneficial early alarm for effective immune clearance, or conversely, may cause hyperinflammation and tissue injury during severe invasive infection. Although traditionally considered an extracellular pathogen, GAS can invade and replicate within macrophages using specific molecular mechanisms to resist phagolysosomal and xenophagic killing. Unraveling GAS-macrophage encounters may reveal new treatment options for this leading agent of infection-associated mortality. [Formula: see text].
Keywords: Group A Streptococcus (GAS); IL-1β signaling; NLRP3 inflammasome; Toll-like receptors; intracellular survival; macrophage; phagocytosis; xenophagy.
Conflict of interest statement
This work was supported by National Institutes of Health grants number AI077780 and AI096837. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures

References
-
- Ralph AP, Carapetis JR. Group A streptococcal diseases and their global burden. Curr. Top. Microbiol. Immunol. 2013;368:1–27. - PubMed
-
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005;5:685–694. - PubMed
-
- Cole JN, Barnett TC, Nizet V, Walker MJ. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 2011;9:724–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
