Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma
- PMID: 29226797
- PMCID: PMC5882485
- DOI: 10.1056/NEJMoa1707447
Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma
Abstract
Background: In a phase 1 trial, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, showed efficacy in patients with refractory large B-cell lymphoma after the failure of conventional therapy.
Methods: In this multicenter, phase 2 trial, we enrolled 111 patients with diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, or transformed follicular lymphoma who had refractory disease despite undergoing recommended prior therapy. Patients received a target dose of 2×106 anti-CD19 CAR T cells per kilogram of body weight after receiving a conditioning regimen of low-dose cyclophosphamide and fludarabine. The primary end point was the rate of objective response (calculated as the combined rates of complete response and partial response). Secondary end points included overall survival, safety, and biomarker assessments.
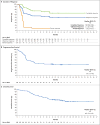
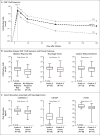
Results: Among the 111 patients who were enrolled, axi-cel was successfully manufactured for 110 (99%) and administered to 101 (91%). The objective response rate was 82%, and the complete response rate was 54%.With a median follow-up of 15.4 months, 42% of the patients continued to have a response, with 40% continuing to have a complete response. The overall rate of survival at 18 months was 52%. The most common adverse events of grade 3 or higher during treatment were neutropenia (in 78% of the patients), anemia (in 43%), and thrombocytopenia (in 38%). Grade 3 or higher cytokine release syndrome and neurologic events occurred in 13% and 28% of the patients, respectively. Three of the patients died during treatment. Higher CAR T-cell levels in blood were associated with response.
Conclusions: In this multicenter study, patients with refractory large B-cell lymphoma who received CAR T-cell therapy with axi-cel had high levels of durable response, with a safety profile that included myelosuppression, the cytokine release syndrome, and neurologic events. (Funded by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program; ZUMA-1 ClinicalTrials.gov number, NCT02348216 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
A Milestone for CAR T Cells.N Engl J Med. 2017 Dec 28;377(26):2593-2596. doi: 10.1056/NEJMe1714680. Epub 2017 Dec 10. N Engl J Med. 2017. PMID: 29226781 No abstract available.
-
Haematological cancer: Favourable outcomes with CAR T cells.Nat Rev Clin Oncol. 2018 Feb;15(2):65. doi: 10.1038/nrclinonc.2017.208. Epub 2018 Jan 3. Nat Rev Clin Oncol. 2018. PMID: 29297506 No abstract available.
-
CAR T-Cell Therapy in Large B-Cell Lymphoma.N Engl J Med. 2018 Mar 15;378(11):1065. doi: 10.1056/NEJMc1800913. N Engl J Med. 2018. PMID: 29542306 No abstract available.
-
Immune Therapies for Lymphomas: A Disruptive Technology With Opportunities for Radiation.Int J Radiat Oncol Biol Phys. 2018 Dec 1;102(5):1396-1399. doi: 10.1016/j.ijrobp.2018.05.079. Int J Radiat Oncol Biol Phys. 2018. PMID: 31014778 No abstract available.
References
-
- Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32. - PubMed
-
- Casulo C, Burack WR, Friedberg JW. Transformed follicular non-Hodgkin lymphoma. Blood. 2015;125:40–7. - PubMed
-
- Ardeshna KM, Kakouros N, Qian W, et al. Conventional second-line salvage chemotherapy regimens are not warranted in patients with malignant lymphomas who have progressive disease after firstline salvage therapy regimens. Br J Haematol. 2005;130:363–72. - PubMed
-
- Hitz F, Connors JM, Gascoyne RD, et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Ann Hematol. 2015;94:1839–43. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
