Cografting astrocytes improves cell therapeutic outcomes in a Parkinson's disease model
- PMID: 29227284
- PMCID: PMC5749514
- DOI: 10.1172/JCI93924
Cografting astrocytes improves cell therapeutic outcomes in a Parkinson's disease model
Abstract
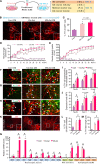
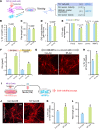
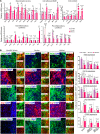
Transplantation of neural progenitor cells (NPCs) is a potential therapy for treating neurodegenerative disorders, but this approach has faced many challenges and limited success, primarily because of inhospitable host brain environments that interfere with enriched neuron engraftment and function. Astrocytes play neurotrophic roles in the developing and adult brain, making them potential candidates for helping with modification of hostile brain environments. In this study, we examined whether astrocytic function could be utilized to overcome the current limitations of cell-based therapies in a murine model of Parkinson's disease (PD) that is characterized by dopamine (DA) neuron degeneration in the midbrain. We show here that cografting astrocytes, especially those derived from the midbrain, remarkably enhanced NPC-based cell therapeutic outcomes along with robust DA neuron engraftment in PD rats for at least 6 months after transplantation. We further show that engineering of donor astrocytes with Nurr1 and Foxa2, transcription factors that were recently reported to polarize harmful immunogenic glia into the neuroprotective form, further promoted the neurotrophic actions of grafted astrocytes in the cell therapeutic approach. Collectively, these findings suggest that cografting astrocytes could be a potential strategy for successful cell therapeutic outcomes in neurodegenerative disorders.
Keywords: Neuronal stem cells; Neuroscience; Parkinson’s disease; Transplantation.
Conflict of interest statement
Figures







Comment in
-
Creating a graft-friendly environment for stem cells in diseased brains.J Clin Invest. 2018 Jan 2;128(1):116-119. doi: 10.1172/JCI98490. Epub 2017 Dec 11. J Clin Invest. 2018. PMID: 29227287 Free PMC article.

