POT1 inhibits the efficiency but promotes the fidelity of nonhomologous end joining at non-telomeric DNA regions
- PMID: 29227966
- PMCID: PMC5764391
- DOI: 10.18632/aging.101339
POT1 inhibits the efficiency but promotes the fidelity of nonhomologous end joining at non-telomeric DNA regions
Abstract
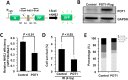
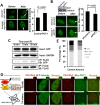
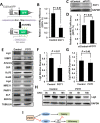
Robust DNA double strand break (DSB) repair and stabilized telomeres help maintain genome integrity, preventing the onset of aging or tumorigenesis. POT1 is one of the six factors in the shelterin complex, which protects telomeres from being recognized as DNA damages. TRF1 and TRF2, two other shelterin proteins, have been shown to participate in DNA DSB repair at non-telomeric regions, but whether POT1, which binds to single strand telomeric DNA at chromosomal ends, is involved in DNA DSB repair has not been assessed. Here we found that POT1 arrives at DNA damage sites upon the occurrence of DNA DSBs. It suppresses the efficiency of nonhomologous end joining (NHEJ), the major pathway for fixing DNA DSBs in mammals, but surprisingly promotes NHEJ fidelity. Mechanistic studies indicate that POT1 facilitates the recruitment of Artemis, which is a nuclease and promotes fidelity of NHEJ, to DNA damage sites. In addition, we found that overexpression of POT1 inhibits the protein stability of Lig3, which is the major regulator of alternative NHEJ (alt-NHEJ), therefore suppressing the efficiency of alt-NHEJ. Taken together we propose that POT1 is a key factor regulating the balance between the efficiency and fidelity of NHEJ at non-telomeric DNA regions.
Keywords: Artemis; DNA Lig3; DNA double strand break repair; NHEJ efficiency; NHEJ fidelity; POT1.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–57. https://doi.org/10.1146/annurev.biochem.77.061306.125255 - DOI - PubMed
-
- Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. https://doi.org/10.1146/annurev.biochem.052308.093131 - DOI - PMC - PubMed
-
- Haber JE. Partners and pathwaysrepairing a double-strand break. Trends Genet. 2000;16:259–64. https://doi.org/10.1016/S0168-9525(00)02022-9 - DOI - PubMed
-
- Yan CT, Boboila C, Souza EK, Franco S, Hickernell TR, Murphy M, Gumaste S, Geyer M, Zarrin AA, Manis JP, Rajewsky K, Alt FW. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–82. https://doi.org/10.1038/nature06020 - DOI - PubMed
-
- Wang M, Wu W, Wu W, Rosidi B, Zhang L, Wang H, Iliakis G. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34:6170–82. https://doi.org/10.1093/nar/gkl840 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

