Thalamocortical control of propofol phase-amplitude coupling
- PMID: 29227992
- PMCID: PMC5739502
- DOI: 10.1371/journal.pcbi.1005879
Thalamocortical control of propofol phase-amplitude coupling
Abstract
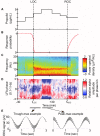
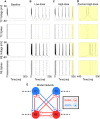
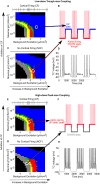
The anesthetic propofol elicits many different spectral properties on the EEG, including alpha oscillations (8-12 Hz), Slow Wave Oscillations (SWO, 0.1-1.5 Hz), and dose-dependent phase-amplitude coupling (PAC) between alpha and SWO. Propofol is known to increase GABAA inhibition and decrease H-current strength, but how it generates these rhythms and their interactions is still unknown. To investigate both generation of the alpha rhythm and its PAC to SWO, we simulate a Hodgkin-Huxley network model of a hyperpolarized thalamus and corticothalamic inputs. We find, for the first time, that the model thalamic network is capable of independently generating the sustained alpha seen in propofol, which may then be relayed to cortex and expressed on the EEG. This dose-dependent sustained alpha critically relies on propofol GABAA potentiation to alter the intrinsic spindling mechanisms of the thalamus. Furthermore, the H-current conductance and background excitation of these thalamic cells must be within specific ranges to exhibit any intrinsic oscillations, including sustained alpha. We also find that, under corticothalamic SWO UP and DOWN states, thalamocortical output can exhibit maximum alpha power at either the peak or trough of this SWO; this implies the thalamus may be the source of propofol-induced PAC. Hyperpolarization level is the main determinant of whether the thalamus exhibits trough-max PAC, which is associated with lower propofol dose, or peak-max PAC, associated with higher dose. These findings suggest: the thalamus generates a novel rhythm under GABAA potentiation such as under propofol, its hyperpolarization may determine whether a patient experiences trough-max or peak-max PAC, and the thalamus is a critical component of propofol-induced cortical spectral phenomena. Changes to the thalamus may be a critical part of how propofol accomplishes its effects, including unconsciousness.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: ENB and PLP are inventors on patents pending on anesthetic brain monitoring that have been licensed by Massachusetts General Hospital to Masimo Corporation. ENB and PLP have received speaker’s honoraria from and ENB has consulted for Masimo Corporation.
Figures






References
-
- Miller RD, editor. Miller’s anesthesia. Eighth edition Philadelphia, PA: Elsevier/Saunders; 2015.
-
- Ishizawa Y, Ahmed OJ, Patel SR, Gale JT, Sierra-Mercado D, Brown EN, et al. Dynamics of Propofol-Induced Loss of Consciousness Across Primate Neocortex. J Neurosci. 2016;36: 7718–7726. doi: 10.1523/JNEUROSCI.4577-15.2016 - DOI - PMC - PubMed
-
- Brown EN, Purdon PL, Van Dort CJ. General Anesthesia and Altered States of Arousal: A Systems Neuroscience Analysis. Annu Rev Neurosci. 2011;34: 601–628. doi: 10.1146/annurev-neuro-060909-153200 - DOI - PMC - PubMed
-
- Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci. 2012;109: E3377–E3386. doi: 10.1073/pnas.1210907109 - DOI - PMC - PubMed
-
- Hanrahan SJ, Greger B, Parker RA, Ogura T, Obara S, Egan TD, et al. The Effects of Propofol on Local Field Potential Spectra, Action Potential Firing Rate, and Their Temporal Relationship in Humans and Felines. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00136 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

