Randomized Controlled Trial of Daily Text Messages to Support Adherence to Preexposure Prophylaxis in Individuals at Risk for Human Immunodeficiency Virus: The TAPIR Study
- PMID: 29228144
- PMCID: PMC6248545
- DOI: 10.1093/cid/cix1055
Randomized Controlled Trial of Daily Text Messages to Support Adherence to Preexposure Prophylaxis in Individuals at Risk for Human Immunodeficiency Virus: The TAPIR Study
Abstract
Background: Adherence is critical for efficacy of tenofovir disoproxil fumarate/emtricitabine (FTC) as preexposure prophylaxis (PrEP).
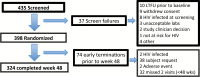
Methods: Between February 2013 and February 2016, 398 men who have sex with men and transgender women were randomized 1:1 to receive individualized texting for adherence building (iTAB) or standard care (SoC) for 48 weeks. The primary endpoint was dried blood spot (DBS) tenofovir diphosphate (TFV-DP) concentrations at both week 12 and the last on-drug visit of >719 fmol/punch (ie, adequate adherence). Secondary outcomes included DBS TFV-DP concentrations of >1246 fmol/punch (ie, near-perfect adherence) and plasma FTC >350 ng/mL (consistent with dosing within the past 24 hours).
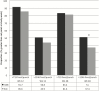
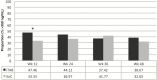
Results: Concentrations >719 fmol/punch of TFV-DP were found in 88.6% of participants at week 12 and 82.5% at week 48. For the primary endpoint, the study arms did not differ (72.0% in iTAB and 69.2% in SoC; P > .05). For the secondary composite endpoint of >1246 fmol/punch the iTAB arm was superior to SoC (33.5% vs 24.8%; P = .06), reaching statistical significance when adjusting for age (odds ratio, 1.56 [95% confidence interval, 1.00-2.42]; P < .05). At week 48, iTAB was superior to SoC for near-perfect adherence (51.0% vs 37.4%; P = .02). At week 12, iTAB was superior to SoC for dosing in past 24 hours by plasma FTC (47.5% vs 33.3%; P = .007), but not at weeks 24, 36, and 48 (all P > .05).
Conclusions: Automated text messaging is a low-burden tool that improves durability of near-perfect PrEP adherence.
Clinical trials registration: NCT01761643.
Figures
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA et al. ; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. - PubMed
-
- Gandhi M, Glidden DV, Liu A et al. ; iPrEx Study Team. Strong correlation between concentrations of tenofovir (TFV) emtricitabine (FTC) in hair and TFV diphosphate and FTC triphosphate in dried blood spots in the iPrEx open label extension: implications for pre-exposure prophylaxis adherence monitoring. J Infect Dis 2015; 212:1402–6. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

