Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis
- PMID: 29228214
- PMCID: PMC5837360
- DOI: 10.1093/brain/awx315
Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis
Erratum in
-
Corrigendum.Brain. 2018 Apr 1;141(4):e33. doi: 10.1093/brain/awy043. Brain. 2018. PMID: 29506215 Free PMC article. No abstract available.
Abstract
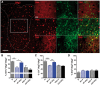
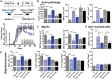
Oestrogen treatments are neuroprotective in a variety of neurodegenerative disease models. Selective oestrogen receptor modifiers are needed to optimize beneficial effects while minimizing adverse effects to achieve neuroprotection in chronic diseases. Oestrogen receptor beta (ERβ) ligands are potential candidates. In the multiple sclerosis model chronic experimental autoimmune encephalomyelitis, ERβ-ligand treatment is neuroprotective, but mechanisms underlying this neuroprotection remain unclear. Specifically, whether there are direct effects of ERβ-ligand on CD11c+ microglia, myeloid dendritic cells or macrophages in vivo during disease is unknown. Here, we generated mice with ERβ deleted from CD11c+ cells to show direct effects of ERβ-ligand treatment in vivo on these cells to mediate neuroprotection during experimental autoimmune encephalomyelitis. Further, we use bone marrow chimeras to show that ERβ in peripherally derived myeloid cells, not resident microglia, are the CD11c+ cells mediating this protection. CD11c+ dendritic cell and macrophages isolated from the central nervous system of wild-type experimental autoimmune encephalomyelitis mice treated with ERβ-ligand expressed less iNOS and T-bet, but more IL-10, and this treatment effect was lost in mice with specific deletion of ERβ in CD11c+ cells. Also, we extend previous reports of ERβ-ligand’s ability to enhance remyelination through a direct effect on oligodendrocytes by showing that the immunomodulatory effect of ERβ-ligand acting on CD11c+ cells is necessary to permit the maturation of oligodendrocytes. Together these results demonstrate that targeting ERβ signalling pathways in CD11c+ myeloid cells is a novel strategy for regulation of the innate immune system in neurodegenerative diseases. To our knowledge, this is the first report showing how direct effects of a candidate neuroprotective treatment on two distinct cell lineages (bone marrow derived myeloid cells and oligodendrocytes) can have complementary neuroprotective effects in vivo.awx315media15688130498001.
Keywords: experimental autoimmune encephalomyelitis; macrophage; multiple sclerosis; neuroprotection; oestrogen receptor beta.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures






Similar articles
-
Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis.J Neurosci. 2006 Jun 21;26(25):6823-33. doi: 10.1523/JNEUROSCI.0453-06.2006. J Neurosci. 2006. PMID: 16793889 Free PMC article.
-
Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis.J Neuroinflammation. 2016 Aug 26;13(1):207. doi: 10.1186/s12974-016-0686-4. J Neuroinflammation. 2016. PMID: 27566665 Free PMC article.
-
Erythropoietin reduces experimental autoimmune encephalomyelitis severity via neuroprotective mechanisms.J Neuroinflammation. 2017 Oct 13;14(1):202. doi: 10.1186/s12974-017-0976-5. J Neuroinflammation. 2017. PMID: 29029628 Free PMC article.
-
Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: Estrogen receptor ligand effects.J Steroid Biochem Mol Biol. 2016 Jun;160:43-52. doi: 10.1016/j.jsbmb.2016.01.006. Epub 2016 Jan 14. J Steroid Biochem Mol Biol. 2016. PMID: 26776441 Free PMC article. Review.
-
Neuronal injury in chronic CNS inflammation.Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review.
Cited by
-
Sex Hormones and Their Effects on Ocular Disorders and Pathophysiology: Current Aspects and Our Experience.Int J Mol Sci. 2022 Mar 17;23(6):3269. doi: 10.3390/ijms23063269. Int J Mol Sci. 2022. PMID: 35328690 Free PMC article. Review.
-
Impact of sex hormones on immune function and multiple sclerosis development.Immunology. 2019 Jan;156(1):9-22. doi: 10.1111/imm.13004. Epub 2018 Oct 11. Immunology. 2019. PMID: 30222193 Free PMC article. Review.
-
Bu Shen Yi Sui Capsule Alleviates Neuroinflammation and Demyelination by Promoting Microglia toward M2 Polarization, Which Correlates with Changes in miR-124 and miR-155 in Experimental Autoimmune Encephalomyelitis.Oxid Med Cell Longev. 2021 Mar 16;2021:5521503. doi: 10.1155/2021/5521503. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33815654 Free PMC article.
-
Sexual Dimorphism in Neurodegenerative Diseases and in Brain Ischemia.Biomolecules. 2022 Dec 22;13(1):26. doi: 10.3390/biom13010026. Biomolecules. 2022. PMID: 36671411 Free PMC article. Review.
-
The Adaptive Immune System in Multiple Sclerosis: An Estrogen-Mediated Point of View.Cells. 2019 Oct 19;8(10):1280. doi: 10.3390/cells8101280. Cells. 2019. PMID: 31635066 Free PMC article. Review.
References
-
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid dendritic cells presenting endogenous myelin peptides ‘preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol 2007; 8: 172–80. - PubMed
-
- Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol 2008; 508: 687–710. - PubMed
-
- Clarkson BD, Walker A, Harris MG, Rayasam A, Sandor M, Fabry Z. CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression. J Immunol 2015; 194: 531–41. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

