Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis
- PMID: 29228214
- PMCID: PMC5837360
- DOI: 10.1093/brain/awx315
Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis
Erratum in
-
Corrigendum.Brain. 2018 Apr 1;141(4):e33. doi: 10.1093/brain/awy043. Brain. 2018. PMID: 29506215 Free PMC article. No abstract available.
Abstract
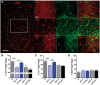
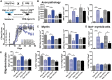
Oestrogen treatments are neuroprotective in a variety of neurodegenerative disease models. Selective oestrogen receptor modifiers are needed to optimize beneficial effects while minimizing adverse effects to achieve neuroprotection in chronic diseases. Oestrogen receptor beta (ERβ) ligands are potential candidates. In the multiple sclerosis model chronic experimental autoimmune encephalomyelitis, ERβ-ligand treatment is neuroprotective, but mechanisms underlying this neuroprotection remain unclear. Specifically, whether there are direct effects of ERβ-ligand on CD11c+ microglia, myeloid dendritic cells or macrophages in vivo during disease is unknown. Here, we generated mice with ERβ deleted from CD11c+ cells to show direct effects of ERβ-ligand treatment in vivo on these cells to mediate neuroprotection during experimental autoimmune encephalomyelitis. Further, we use bone marrow chimeras to show that ERβ in peripherally derived myeloid cells, not resident microglia, are the CD11c+ cells mediating this protection. CD11c+ dendritic cell and macrophages isolated from the central nervous system of wild-type experimental autoimmune encephalomyelitis mice treated with ERβ-ligand expressed less iNOS and T-bet, but more IL-10, and this treatment effect was lost in mice with specific deletion of ERβ in CD11c+ cells. Also, we extend previous reports of ERβ-ligand’s ability to enhance remyelination through a direct effect on oligodendrocytes by showing that the immunomodulatory effect of ERβ-ligand acting on CD11c+ cells is necessary to permit the maturation of oligodendrocytes. Together these results demonstrate that targeting ERβ signalling pathways in CD11c+ myeloid cells is a novel strategy for regulation of the innate immune system in neurodegenerative diseases. To our knowledge, this is the first report showing how direct effects of a candidate neuroprotective treatment on two distinct cell lineages (bone marrow derived myeloid cells and oligodendrocytes) can have complementary neuroprotective effects in vivo.awx315media15688130498001.
Keywords: experimental autoimmune encephalomyelitis; macrophage; multiple sclerosis; neuroprotection; oestrogen receptor beta.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures






References
-
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid dendritic cells presenting endogenous myelin peptides ‘preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol 2007; 8: 172–80. - PubMed
-
- Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol 2008; 508: 687–710. - PubMed
-
- Clarkson BD, Walker A, Harris MG, Rayasam A, Sandor M, Fabry Z. CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression. J Immunol 2015; 194: 531–41. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

