A Randomized, Double-Blind, Placebo- and Active Comparator-Controlled Phase I Study of Analgesic/Antihyperalgesic Properties of ASP8477, a Fatty Acid Amide Hydrolase Inhibitor, in Healthy Female Subjects
- PMID: 29228247
- PMCID: PMC5998989
- DOI: 10.1093/pm/pnx281
A Randomized, Double-Blind, Placebo- and Active Comparator-Controlled Phase I Study of Analgesic/Antihyperalgesic Properties of ASP8477, a Fatty Acid Amide Hydrolase Inhibitor, in Healthy Female Subjects
Abstract
Objectives: To evaluate the analgesic/antihyperalgesic effect of ASP8477.
Design: Randomized, double-blind, double-dummy, cross-over, placebo- and active comparator-controlled study.
Setting: HPR Dr. Schaffler GmbH, Munich, Germany.
Subjects: Healthy female subjects aged 18-65 years.
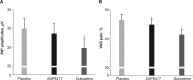
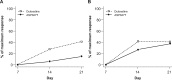
Methods: Eligible subjects were randomly assigned to one of six treatment sequences and received multiple ascending doses of ASP8477, duloxetine, and placebo over three treatment periods (each consisting of 21-day dosing separated by 14-day washout periods). On the last day of each dose level, laser evoked potentials (LEPs) and visual analog scales (VAS pain) on capsaicin-treated skin at baseline and at multiple postdose time points were assessed. The primary end point was the difference in LEP N2-P2 peak-to-peak (PtP) amplitudes for ASP8477 100 mg vs placebo.
Results: Twenty-five subjects were randomized. In all subjects, LEP N2-P2 PtP amplitudes were numerically lower for ASP8477 100 mg vs placebo (P = 0.0721); in subjects who demonstrated positive capsaicin skin effects, a greater mean difference of -2.24 µV (P = 0.0146) was observed. Across all doses, LEP N2-P2 PtP amplitudes were lower for duloxetine compared with ASP8477 (mean difference -3.80 µV; P < 0.0001) or placebo (mean difference -5.21 µV; P < 0.0001). The effect of ASP8477 (all doses) on down-scoring the VAS pain score was significant compared with placebo (mean difference -2.55%; P < 0.0007).
Conclusions: ASP8477 was well tolerated in this study. Analysis of all subjects did not demonstrate a significant difference in LEP for ASP8477 100 mg over placebo but did in subjects who demonstrated positive capsaicin skin effects.
Figures


References
-
- Lodola A, Castelli R, Mor M, et al. Fatty acid amide hydrolase inhibitors: A patent review (2009-2014). Expert Opin Ther Pat 2015;25(11):1247–66. - PubMed
-
- Ahn K, Smith SE, Liimatta MB, et al. Mechanistic and pharmacological characterization of PF-04457845: A highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther 2011;338(1):114–24.http://dx.doi.org/10.1124/jpet.111.180257 - DOI - PMC - PubMed
-
- Caprioli A, Coccurello R, Rapino C, et al. The novel reversible fatty acid amide hydrolase inhibitor ST4070 increases endocannabinoid brain levels and counteracts neuropathic pain in different animal models. J Pharmacol Exp Ther 2012;342(1):188–95.http://dx.doi.org/10.1124/jpet.111.191403 - DOI - PubMed
-
- Clapper JR, Moreno-Sanz G, Russo R, et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci 2010;13(10):1265–70.http://dx.doi.org/10.1038/nn.2632 - DOI - PMC - PubMed
-
- Jhaveri MD, Richardson D, Kendall DA, et al. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci 2006;26(51):13318–27.http://dx.doi.org/10.1523/JNEUROSCI.3326-06.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

