Leukocyte Telomere Length at Birth and During the Early Life of Children Exposed to but Uninfected With HIV After In Utero Exposure to Antiretrovirals
- PMID: 29228317
- PMCID: PMC5853286
- DOI: 10.1093/infdis/jix618
Leukocyte Telomere Length at Birth and During the Early Life of Children Exposed to but Uninfected With HIV After In Utero Exposure to Antiretrovirals
Abstract
Background: Maternal combination antiretroviral therapy (cART) during pregnancy could impact the health of human immunodeficiency virus (HIV)-exposed, HIV-uninfected (HEU) children, because some antiretrovirals cross the placenta and can inhibit telomerase. Our objective was to compare leukocyte telomere length (LTL) in HEU children and HIV-unexposed, HIV-uninfected (HUU) children at birth and in early life and to investigate any relationship with cART exposure.
Methods: HEU and HUU children's blood LTL was compared cross-sectionally at birth, and during the first three years of life. Longitudinal HEU LTL dynamics was evaluated over that same period.
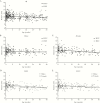
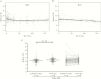
Results: At birth, the LTL in HEU children (n = 114) was not shorter than that in HUU children (n = 86), but female infants had longer LTL than male infants. Maternal cART (duration or type) showed no association with shorter infant LTL. Among 214 HEU children age- and sex-matched at a 1:1 ratio to HUU children, LTL declined similarly in both groups. In a longitudinal analysis, LTL attrition in HEU children was rapid from birth to 1 year of age and gradual thereafter. Zidovudine prophylaxis did not significantly alter LTL.
Conclusions: Our results indicate that from birth to 3 years of age, the LTL in HEU children is not negatively affected by exposure to maternal HIV infection and cART, at least not to the regimens used within this Canadian cohort, a reassuring finding.
Keywords: HIV-exposed uninfected; Leukocyte telomere length; combination antiretroviral therapy; longitudinal LTL attrition; maternal smoking during pregnancy.
© The Author(s) 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Association between zidovudine-containing antiretroviral therapy exposure in utero and leukocyte telomere length at birth.AIDS. 2019 Nov 1;33(13):2091-2096. doi: 10.1097/QAD.0000000000002317. AIDS. 2019. PMID: 31335808 Free PMC article.
-
Leukocyte telomere length in HIV-infected and HIV-exposed uninfected children: shorter telomeres with uncontrolled HIV viremia.PLoS One. 2012;7(7):e39266. doi: 10.1371/journal.pone.0039266. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22815702 Free PMC article. Clinical Trial.
-
Leukocyte telomere length in HIV-infected pregnant women treated with antiretroviral drugs during pregnancy and their uninfected infants.J Acquir Immune Defic Syndr. 2012 Aug 15;60(5):495-502. doi: 10.1097/QAI.0b013e31825aa89c. J Acquir Immune Defic Syndr. 2012. PMID: 22580562
-
Health and survival of HIV perinatally exposed but uninfected children born to HIV-infected mothers.Curr Opin HIV AIDS. 2016 Sep;11(5):465-476. doi: 10.1097/COH.0000000000000300. Curr Opin HIV AIDS. 2016. PMID: 27716731 Review.
-
Mortality risk and associated factors in HIV-exposed, uninfected children.Trop Med Int Health. 2016 Jun;21(6):720-34. doi: 10.1111/tmi.12695. Epub 2016 Apr 19. Trop Med Int Health. 2016. PMID: 27091659 Free PMC article. Review.
Cited by
-
Elevated Blood Mitochondrial DNA in Early Life Among Uninfected Children Exposed to Human Immunodeficiency Virus and Combination Antiretroviral Therapy in utero.J Infect Dis. 2021 Feb 24;223(4):621-631. doi: 10.1093/infdis/jiaa410. J Infect Dis. 2021. PMID: 32638023 Free PMC article.
-
Longitudinal Follow-Up of Blood Telomere Length in HIV-Exposed Uninfected Children Having Received One Year of Lopinavir/Ritonavir or Lamivudine as Prophylaxis.Children (Basel). 2021 Sep 10;8(9):796. doi: 10.3390/children8090796. Children (Basel). 2021. PMID: 34572228 Free PMC article.
-
Association between zidovudine-containing antiretroviral therapy exposure in utero and leukocyte telomere length at birth.AIDS. 2019 Nov 1;33(13):2091-2096. doi: 10.1097/QAD.0000000000002317. AIDS. 2019. PMID: 31335808 Free PMC article.
-
Biological Aging and Immune Senescence in Children with Perinatally Acquired HIV.J Immunol Res. 2020 May 16;2020:8041616. doi: 10.1155/2020/8041616. eCollection 2020. J Immunol Res. 2020. PMID: 32509884 Free PMC article. Review.
-
Biomarkers of Aging in HIV-Infected Children on Suppressive Antiretroviral Therapy.J Acquir Immune Defic Syndr. 2018 Aug 15;78(5):549-556. doi: 10.1097/QAI.0000000000001714. J Acquir Immune Defic Syndr. 2018. PMID: 29771780 Free PMC article.
References
-
- Johnson AS, Hess K, Hu S et al. . Monitoring selected national HIV prevention and care objectives by using HIV surveillance data United States and 6 dependent areas, 2014. HIV Surveill Rep 2015; 21:1–87.
-
- Forbes JC, Alimenti AM, Singer J et al. . Canadian Pediatric AIDS Research Group (CPARG) A national review of vertical HIV transmission. AIDS 2012; 26:757–63. - PubMed
-
- World Health Organization (WHO). Guidelines guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: WHO, 2015. - PubMed
-
- Arab K, Spence RA, Czujoj-Shulman N, Abenhaim HA. Pregnancy outcomes in HIV-positive women: a retrospective cohort study. Arch Gynecol Obstet 2017; 295:1–8. - PubMed
-
- Desmonde S, Goetghebuer T, Thorne C, Leroy V. Health and survival of HIV perinatally exposed but uninfected children born to HIV-infected mothers. Curr Opin HIV AIDS 2016; 11:465–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

