Ammonium glycyrrhizin counteracts liver injury caused by lipopolysaccharide/amoxicillin-clavulanate potassium
- PMID: 29228575
- PMCID: PMC5722527
- DOI: 10.18632/oncotarget.18291
Ammonium glycyrrhizin counteracts liver injury caused by lipopolysaccharide/amoxicillin-clavulanate potassium
Abstract
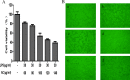
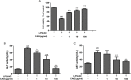
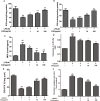
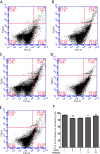
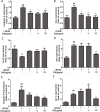
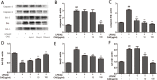
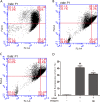
We treated isolated chicken primary hepatocytes with lipopolysaccharide/amoxicillin clavulanate potassium (LPS/AC) to model liver injury and investigate its underlying mechanisms. We also used this model to assess the cytoprotective effects of compound ammonium glycyrrhizin (CAG) in vitro. LPS/AC-induced injury decreased cell viability and increased the activity of serum aspartate transaminase and alanine transaminase. Levels of superoxide dismutase, glutathione, and glutathione peroxidase were lower than control, while levels of the oxidative product malondialdehyde and reactive oxygen species were higher. Treatment with CAG for 24 h ameliorated these changes. Caspase-3 activity assays and flow cytometry revealed increased apoptosis in the model group. However, apoptosis decreased after CAG treatment, as confirmed by Hoechst 33342 staining. We also observed changes in mitochondrial ultrastructure. Real-time PCR and western blot analyses showed that CAG treatment downregulated LPS/AC-induced RNA expression of caspase-3, caspase-9, bax, cytochrome c, and fas, and upregulated the expression of bcl-2. Mitochondrial cytochrome c was released into the cytosol and the inner mitochondrial membrane potential (ΔΨm) was decreased. Our results highlight CAG as a potential therapeutic agent to counteract LPS/AC-induced liver injury.
Keywords: amoxicillin clavulanate potassium; antioxidant; chicken primary hepatocytes; compound ammonium glycyrrhizin; lipopolysaccharide.
Conflict of interest statement
CONFLICTS OF INTEREST None to declare.
Figures



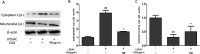

Similar articles
-
Compound Ammonium Glycyrrhizin Protects Hepatocytes from Injury Induced by Lipopolysaccharide/Florfenicol through a Mitochondrial Pathway.Molecules. 2018 Sep 17;23(9):2378. doi: 10.3390/molecules23092378. Molecules. 2018. PMID: 30227687 Free PMC article.
-
Protective effects of compound ammonium glycyrrhizin, L‑arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes.Mol Med Rep. 2018 Sep;18(3):2551-2560. doi: 10.3892/mmr.2018.9285. Epub 2018 Jul 16. Mol Med Rep. 2018. PMID: 30015927 Free PMC article.
-
Composite ammonium glycyrrhizin has hepatoprotective effects in chicken hepatocytes with lipopolysaccharide/enrofloxacin-induced injury.Exp Ther Med. 2020 Nov;20(5):52. doi: 10.3892/etm.2020.9180. Epub 2020 Sep 4. Exp Ther Med. 2020. PMID: 32952642 Free PMC article.
-
Compound ammonium glycyrrhizin protects hepatocytes from injury induced by lipopolysaccharide/florfenicol through oxidative stress and a MAPK pathway.Comp Biochem Physiol C Toxicol Pharmacol. 2019 Nov;225:108585. doi: 10.1016/j.cbpc.2019.108585. Epub 2019 Aug 6. Comp Biochem Physiol C Toxicol Pharmacol. 2019. PMID: 31398390
-
Amoxicillin-clavulanate-induced Granulomatous Hepatitis: Case Report and Review of the Literature.J Clin Transl Hepatol. 2019 Sep 28;7(3):280-283. doi: 10.14218/JCTH.2019.00027. Epub 2019 Jul 26. J Clin Transl Hepatol. 2019. PMID: 31608221 Free PMC article. Review.
Cited by
-
Compound Ammonium Glycyrrhizin Protects Hepatocytes from Injury Induced by Lipopolysaccharide/Florfenicol through a Mitochondrial Pathway.Molecules. 2018 Sep 17;23(9):2378. doi: 10.3390/molecules23092378. Molecules. 2018. PMID: 30227687 Free PMC article.
-
Protective effects of compound ammonium glycyrrhizin, L‑arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes.Mol Med Rep. 2018 Sep;18(3):2551-2560. doi: 10.3892/mmr.2018.9285. Epub 2018 Jul 16. Mol Med Rep. 2018. PMID: 30015927 Free PMC article.
References
-
- Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi https://doi.org/10.1016/S0952-7915(99)80004-1. - DOI - PubMed
-
- Jeong YI, Jung ID, Lee CM, Chang JH, Chun SH, Noh KT, Jeong SK, Shin YK, Lee WS, Kang MS, Lee SY, Lee JD, Park YM. The novel role of platelet-activating factor in protecting mice against lipopolysaccharide-induced endotoxic shock. PLoS One. 2009;4:e6503. https://doi.org/10.1371/journal.pone.0006503. - DOI - PMC - PubMed
-
- Thirunavukkarasu C, Uemura T, Wang LF, Watkins SC, Gandhi CR. Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J Cell Physiol. 2005;204:654–65. https://doi.org/10.1002/jcp.20366. - DOI - PubMed
-
- Michie HR, Manogue KR, Spriggs DR, Revhaug A, O’Dwyer S, Dinarello CA, Cerami A, Wolff SM, Wilmore DW. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–86. https://doi.org/10.1056/NEJM198806093182301. - DOI - PubMed
-
- De Valle MB, Av Klinteberg V, Alem N, Olsson R, Björnsson E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther. 2006;24:1187–95. https://doi.org/10.1111/j.1365-2036.2006.03117.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

