Irisin treatment improves healing of dystrophic skeletal muscle
- PMID: 29228710
- PMCID: PMC5716750
- DOI: 10.18632/oncotarget.21636
Irisin treatment improves healing of dystrophic skeletal muscle
Abstract
Background: Irisin is an exercise induced myokine that is shown to promote browning of adipose tissue and hence, increase energy expenditure. Furthermore, our unpublished results indicate that Irisin improves myogenic differentiation and induces skeletal muscle hypertrophy. Since exercise induced skeletal muscle hypertrophy improves muscle strength, we wanted to investigate if ectopic injection of Irisin peptide improves skeletal muscle function in a mouse model of muscular dystrophy. This utility of Irisin peptide is yet to be studied in animal models.
Methods: In order to test this hypothesis, we expressed and purified recombinant murine Irisin peptide from E. coli. Three- to six-week-old male mdx mice were injected IP with either vehicle (dialysis buffer) or Irisin recombinant peptide for two or four weeks, three times-a-week.
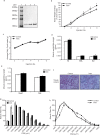
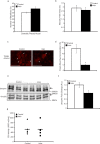
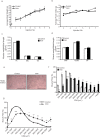
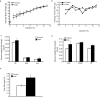
Results: Irisin injection increased muscle weights and enhanced grip strength in mdx mice. Improved muscle strength can be attributed to the significant hypertrophy observed in the Irisin injected mdx mice. Moreover, Irisin treatment resulted in reduced accumulation of fibrotic tissue and myofiber necrosis in mdx mice. In addition, Irisin improved sarcolemmal stability, which is severely compromised in mdx mice.
Conclusion: Irisin injection induced skeletal muscle hypertrophy, improved muscle strength and reduced necrosis and fibrotic tissue in a murine dystrophy model. These results demonstrate the potential therapeutic value of Irisin in muscular dystrophy.
Keywords: FNDC5; dystrophy; irisin; sarcolemmal stability; skeletal muscle.
Conflict of interest statement
CONFLICTS OF INTEREST Musarrat Maisha Reza, Chu Ming Sim, Nathiya Subramaniyam, Xiaojia Ge, Mridula Sharma, Ravi Kambadur and Craig McFarlane declare that they have no conflict of interest.
Figures

Similar articles
-
Effects of moderate aerobic exercise, low-level laser therapy, or their combination on muscles pathology, oxidative stress and irisin levels in the mdx mouse model of Duchenne muscular dystrophy.Lasers Med Sci. 2022 Sep;37(7):2925-2936. doi: 10.1007/s10103-022-03562-8. Epub 2022 Apr 19. Lasers Med Sci. 2022. PMID: 35441320
-
Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy.Nat Commun. 2017 Oct 24;8(1):1104. doi: 10.1038/s41467-017-01131-0. Nat Commun. 2017. PMID: 29062100 Free PMC article.
-
A GDF11/myostatin inhibitor, GDF11 propeptide-Fc, increases skeletal muscle mass and improves muscle strength in dystrophic mdx mice.Skelet Muscle. 2019 May 27;9(1):16. doi: 10.1186/s13395-019-0197-y. Skelet Muscle. 2019. PMID: 31133057 Free PMC article.
-
Crosstalk Between Muscle and Bone Via the Muscle-Myokine Irisin.Curr Osteoporos Rep. 2016 Aug;14(4):132-7. doi: 10.1007/s11914-016-0313-4. Curr Osteoporos Rep. 2016. PMID: 27299471 Review.
-
Multiple Roles in Neuroprotection for the Exercise Derived Myokine Irisin.Front Aging Neurosci. 2021 Apr 16;13:649929. doi: 10.3389/fnagi.2021.649929. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33935687 Free PMC article. Review.
Cited by
-
Exerkines and myokines in aging sarcopenia.Front Endocrinol (Lausanne). 2025 Jul 29;16:1592491. doi: 10.3389/fendo.2025.1592491. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40801027 Free PMC article. Review.
-
Effects of moderate aerobic exercise, low-level laser therapy, or their combination on muscles pathology, oxidative stress and irisin levels in the mdx mouse model of Duchenne muscular dystrophy.Lasers Med Sci. 2022 Sep;37(7):2925-2936. doi: 10.1007/s10103-022-03562-8. Epub 2022 Apr 19. Lasers Med Sci. 2022. PMID: 35441320
-
The Role of Irisin in Exercise-Mediated Bone Health.Front Cell Dev Biol. 2021 May 4;9:668759. doi: 10.3389/fcell.2021.668759. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34017836 Free PMC article. Review.
-
Myokines as Possible Therapeutic Targets in Cancer Cachexia.J Immunol Res. 2018 Oct 22;2018:8260742. doi: 10.1155/2018/8260742. eCollection 2018. J Immunol Res. 2018. PMID: 30426026 Free PMC article. Review.
-
Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration.Int J Mol Sci. 2023 Mar 31;24(7):6551. doi: 10.3390/ijms24076551. Int J Mol Sci. 2023. PMID: 37047523 Free PMC article. Review.
References
-
- Sussman M. Duchenne muscular dystrophy. J Am Acad Orthop Surg. 2002;10:138–51. - PubMed
-
- Menezes MP, North KN. Inherited neuromuscular disorders: pathway to diagnosis. J Paediatr Child Health. 2012;48:458–65. http://doi.org/10.1111/j.1440-1754.2011.02210.x - DOI - PubMed
-
- Yiu EM, Kornberg AJ. Duchenne muscular dystrophy. Neurol India. 2008;56:236–47. - PubMed
-
- Hoffman EP, Monaco AP, Feener CC, Kunkel LM. Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science. 1987;238:347–50. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

