Prohibitin-mediated mitochondrial ubiquitination during spermiogenesis in Chinese mitten crab Eriocheir sinensis
- PMID: 29228727
- PMCID: PMC5716767
- DOI: 10.18632/oncotarget.21961
Prohibitin-mediated mitochondrial ubiquitination during spermiogenesis in Chinese mitten crab Eriocheir sinensis
Abstract
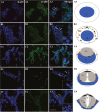
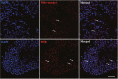
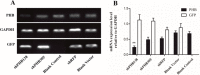
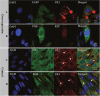
The sperm of Eriocheir sinensis has a cup-shaped nucleus that contains several mitochondria embedded at the opening of the cup. The acrosome vesicle also contains derivants of mitochondria. The mitochondria distribution pattern involves a decrease in the number and changes in the structure and transportation of these organelles. The decreased number of sperm mitochondria is achieved through autophagy or the ubiquitination pathway. Prohibitin (PHB), the mitochondria inner membrane protein, is an evolutionarily highly conserved protein, is closely associated with spermatogenesis and sperm quality control and is also a potential substrate of ubiquitination. However, whether PHB protein mediates the ubiquitination pathway of sperm mitochondria in crustacean animals remains poorly understood. In the present study, we revealed that PHB, a substrate of ubiquitin, participates in the ubiquitination and degradation of mitochondria during spermiogenesis in E. sinensis. To confirm this finding, we used shRNA interference to reduce PHB expression and an overexpression technique to increase PHB expression in vitro. The interference experiment showed that the reduced PHB expression directly affected the polyubiquitination level and mitochondria status, whereas PHB overexpression markedly increased the polyubiquitination level. In vitro experiments also showed that PHB and its ubiquitination decide the fate of mitochondria.
Keywords: Eriocheir sinensis; crustacean; mitochondria; prohibitin; ubiquitination.
Conflict of interest statement
CONFLICTS OF INTEREST The authors confirm that this article has no conflicts of interest.
Figures







Similar articles
-
Mitochondrial Quality Control Mechanisms and the PHB (Prohibitin) Complex.Cells. 2018 Nov 29;7(12):238. doi: 10.3390/cells7120238. Cells. 2018. PMID: 30501123 Free PMC article. Review.
-
Mitochondrial prohibitin and its ubiquitination during crayfish Procambarus clarkii spermiogenesis.Cell Tissue Res. 2015 Feb;359(2):679-692. doi: 10.1007/s00441-014-2044-0. Epub 2014 Nov 25. Cell Tissue Res. 2015. PMID: 25418137
-
The expression pattern and potential functions of PHB in the spermiogenesis of Phascolosoma esculenta.Gene. 2018 Apr 30;652:25-38. doi: 10.1016/j.gene.2018.01.056. Epub 2018 Jan 31. Gene. 2018. PMID: 29360606
-
Mitochondrial prohibitin and its ubiquitination during spermatogenesis of the swimming crab Charybdis japonica.Gene. 2017 Sep 5;627:137-148. doi: 10.1016/j.gene.2017.06.025. Epub 2017 Jun 13. Gene. 2017. PMID: 28627439
-
Prohibitin - At the crossroads of obesity-linked diabetes and cancer.Exp Biol Med (Maywood). 2017 Jun;242(11):1170-1177. doi: 10.1177/1535370217703976. Epub 2017 Apr 11. Exp Biol Med (Maywood). 2017. PMID: 28399645 Free PMC article. Review.
Cited by
-
Characterization of Mitochondrial Prohibitin in Opsariichthys bidens and Its Potential Functions in Spermatogenesis.Int J Mol Sci. 2022 Jun 30;23(13):7295. doi: 10.3390/ijms23137295. Int J Mol Sci. 2022. PMID: 35806298 Free PMC article.
-
OPENER Is a Nuclear Envelope and Mitochondria Localized Protein Required for Cell Cycle Progression in Arabidopsis.Plant Cell. 2019 Jul;31(7):1446-1465. doi: 10.1105/tpc.19.00033. Epub 2019 Apr 25. Plant Cell. 2019. PMID: 31023726 Free PMC article.
-
The Effect of Prohibitins on Mitochondrial Function during Octopus tankahkeei Spermiogenesis.Int J Mol Sci. 2023 Jun 12;24(12):10030. doi: 10.3390/ijms241210030. Int J Mol Sci. 2023. PMID: 37373178 Free PMC article.
-
Mitochondrial Quality Control Mechanisms and the PHB (Prohibitin) Complex.Cells. 2018 Nov 29;7(12):238. doi: 10.3390/cells7120238. Cells. 2018. PMID: 30501123 Free PMC article. Review.
References
-
- Hou CC, Yang WX. Characterization and expression pattern of p53 during spermatogenesis in the Chinese mitten crab Eriocheir sinensis. Mol Biol Rep. 2013;40:1043–1051. - PubMed
-
- Wang YT, Mao H, Hou CC, Sun X, Wang DH, Zhou H, Yang WX. Characterization and expression pattern of KIFC1-like kinesin gene in the testis of the Macrobrachium nipponense with discussion of its relationship with structure lamellar complex (LCx) and acroframosome (AFS) Mol Biol Rep. 2012;39:7591–7598. - PubMed
-
- Fawcett DW. The mammalian spermatozoon. Dev Biol. 1975;44:394–436. - PubMed
-
- Meinhardt A, Wilhelm B, Seitz J. Expression of mitochondrial marker proteins during spermatogenesis. Hum Reprod Update. 1999;5:108–119. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

