Recovery of mrs3Δmrs4Δ Saccharomyces cerevisiae Cells under Iron-Sufficient Conditions and the Role of Fe580
- PMID: 29228768
- PMCID: PMC6468996
- DOI: 10.1021/acs.biochem.7b01034
Recovery of mrs3Δmrs4Δ Saccharomyces cerevisiae Cells under Iron-Sufficient Conditions and the Role of Fe580
Abstract
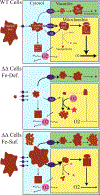
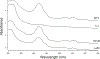
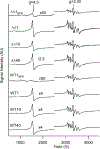
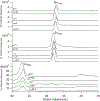
Mrs3 and Mrs4 are mitochondrial inner membrane proteins that deliver an unidentified cytosolic iron species into the matrix for use in iron-sulfur cluster (ISC) and heme biosynthesis. The Mrs3/4 double-deletion strain (ΔΔ) grew slowly in iron-deficient glycerol/ethanol medium but recovered to wild-type (WT) rates in iron-sufficient medium. ΔΔ cells grown under both iron-deficient and iron-sufficient respiring conditions acquired large amounts of iron relative to WT cells, indicating iron homeostatic dysregulation regardless of nutrient iron status. Biophysical spectroscopy (including Mössbauer, electron paramagnetic resonance, and electronic absorption) and bioanalytical methods (liquid chromatography with online inductively coupled plasma mass spectrometry detection) were used to characterize these phenotypes. Anaerobically isolated mitochondria contained a labile iron pool composed of a nonheme high-spin FeII complex with primarily O and N donor ligands, called Fe580. Fe580 likely serves as feedstock for ISC and heme biosynthesis. Mitochondria from respiring ΔΔ cells grown under iron-deficient conditions were devoid of Fe580, ISCs, and hemes; most iron was present as FeIII nanoparticles. O2 likely penetrates the matrix of slow-growing poorly respiring iron-deficient ΔΔ cells and reacts with Fe580 to form nanoparticles, thereby inhibiting ISC and heme biosynthesis. Mitochondria from iron-sufficient ΔΔ cells contained ISCs, hemes, and Fe580 at concentrations comparable to those of WT mitochondria. The matrix of these mutant cells was probably sufficiently anaerobic to protect Fe580 from degradation by O2. An ∼1100 Da manganese complex, an ∼1200 Da zinc complex, and an ∼5000 Da copper species were also present in ΔΔ and WT mitochondrial flow-through solutions. No lower-mass copper complex was evident.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Iron Homeostatic Regulation in Saccharomyces cerevisiae: Introduction to a Computational Modeling Method.Methods Mol Biol. 2024;2839:3-29. doi: 10.1007/978-1-0716-4043-2_1. Methods Mol Biol. 2024. PMID: 39008245 Free PMC article. Review.
-
Mössbauer and LC-ICP-MS investigation of iron trafficking between vacuoles and mitochondria in vma2ΔSaccharomyces cerevisiae.J Biol Chem. 2021 Jan-Jun;296:100141. doi: 10.1074/jbc.RA120.015907. Epub 2020 Dec 6. J Biol Chem. 2021. PMID: 33268384 Free PMC article.
-
Biophysical characterization of the iron in mitochondria from Atm1p-depleted Saccharomyces cerevisiae.Biochemistry. 2009 Oct 13;48(40):9556-68. doi: 10.1021/bi901110n. Biochemistry. 2009. PMID: 19761223 Free PMC article.
-
A mathematical model of iron import and trafficking in wild-type and Mrs3/4ΔΔ yeast cells.BMC Syst Biol. 2019 Feb 21;13(1):23. doi: 10.1186/s12918-019-0702-2. BMC Syst Biol. 2019. PMID: 30791941 Free PMC article.
-
Labile Low-Molecular-Mass Metal Complexes in Mitochondria: Trials and Tribulations of a Burgeoning Field.Biochemistry. 2016 Aug 2;55(30):4140-53. doi: 10.1021/acs.biochem.6b00216. Epub 2016 Jul 19. Biochemistry. 2016. PMID: 27433847 Free PMC article. Review.
Cited by
-
Iron Homeostatic Regulation in Saccharomyces cerevisiae: Introduction to a Computational Modeling Method.Methods Mol Biol. 2024;2839:3-29. doi: 10.1007/978-1-0716-4043-2_1. Methods Mol Biol. 2024. PMID: 39008245 Free PMC article. Review.
-
Recent progress of methods for cuproptosis detection.Front Mol Biosci. 2024 Sep 4;11:1460987. doi: 10.3389/fmolb.2024.1460987. eCollection 2024. Front Mol Biosci. 2024. PMID: 39297074 Free PMC article. Review.
-
CUP1 Metallothionein from Healthy Saccharomyces cerevisiae Colocalizes to the Cytosol and Mitochondrial Intermembrane Space.Biochemistry. 2023 Jan 3;62(1):62-74. doi: 10.1021/acs.biochem.2c00481. Epub 2022 Dec 12. Biochemistry. 2023. PMID: 36503220 Free PMC article.
-
Proteomic strategies to interrogate the Fe-S proteome.Biochim Biophys Acta Mol Cell Res. 2024 Oct;1871(7):119791. doi: 10.1016/j.bbamcr.2024.119791. Epub 2024 Jun 25. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38925478 Review.
-
Splitting the functions of Rim2, a mitochondrial iron/pyrimidine carrier.Mitochondrion. 2019 Jul;47:256-265. doi: 10.1016/j.mito.2018.12.005. Epub 2019 Jan 18. Mitochondrion. 2019. PMID: 30660752 Free PMC article.
References
-
- Lill R, Dutkiewicz R, Freibert SA, Heidenreich T, Mascarenhas J, Netz DJ, Paul VD, Pierik AJ, Richter N, Stuempfig M, Srinivasan V, Stehling O, and Mühlenhoff U (2015) The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron-sulfur proteins. Eur. J. Cell Biol 94, 280–291. - PubMed
-
- Mühlenhoff U, Hoffmann B, Richter N, Rietzschel N, Spantgar F, Stehling O, Uzarska MA, and Lill R (2015) Compartmentalization of iron between mitochondria and the cytosol and its regulation. Eur. J. Cell Biol 94, 292–308. - PubMed
-
- Foury F, and Roganti T (2002) Deletion of the mitochondrial carrier genes MRS3 and MRS4 suppresses mitochondrial iron accumulation in a yeast frataxin-deficient strain. J. Biol. Chem 277, 24475–24483. - PubMed
-
- Mühlenhoff U, Stadler JA, Richhardt N, Seubert A, Eickhorst T, Schweyen RJ, Lill R, and Wiesenberger G (2003) A specific role of the yeast mitochondrial carriers Mrs3/4p in mitochondrial iron acquisition under iron-limiting conditions. J. Biol. Chem 278, 40612–40620. - PubMed
-
- Li LT, and Kaplan J (2004) A mitochondrial-vacuolar signaling pathway in yeast that affects iron and copper metabolism. J. Biol. Chem 279, 33653–33661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

