Nevirapine- versus Efavirenz-based antiretroviral therapy regimens in antiretroviral-naive patients with HIV and Tuberculosis infections in India: a multi-centre study
- PMID: 29228918
- PMCID: PMC5725946
- DOI: 10.1186/s12879-017-2864-0
Nevirapine- versus Efavirenz-based antiretroviral therapy regimens in antiretroviral-naive patients with HIV and Tuberculosis infections in India: a multi-centre study
Abstract
Background: According to World Health Organization (WHO) guidelines, which have also been adopted by the National AIDS Control Organization (NACO), India, Efavirenz-based Anti-Retroviral Therapy (ART) is better in Human-Immunodeficiency-Virus (HIV)-infected patients who are also being treated with Rifampicin-based Anti-Tuberculous Therapy (ATT). However, Efavirenz is much more expensive. We hypothesize that Nevirapine is a cheaper alternative that possesses equal efficacy as Efavirenz in HIV-Tuberculosis (TB) co-infected patients.
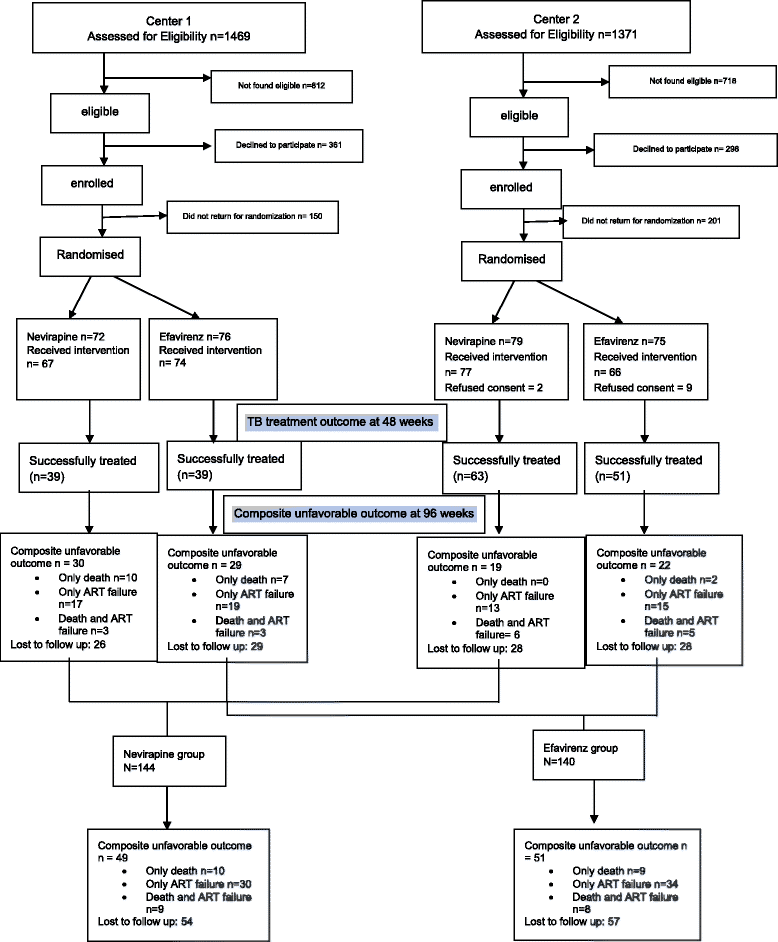
Methods: A parallel open-label randomized clinical trial was conducted at All India Institute of Medical Sciences (AIIMS), New Delhi and National AIDS Research Institute (NARI), Pune. Those who were ART-naïve and co-infected with TB were randomized to receive either Nevirapine (Group 1)- or Efavirenz (Group 2)-based ART along with Rifampicin-based ATT. ATT was begun first in ART-naïve patients according to the NACO guidelines, with a median of 27 days between ATT and ART in both groups. The primary endpoint was a composite unfavourable outcome (death and/or ART failure) at 96 weeks, and the secondary outcome was successful TB treatment at 48 weeks.
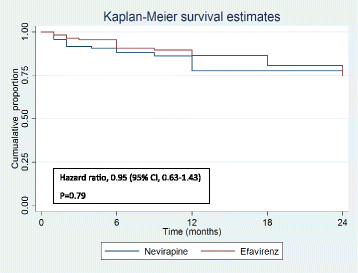
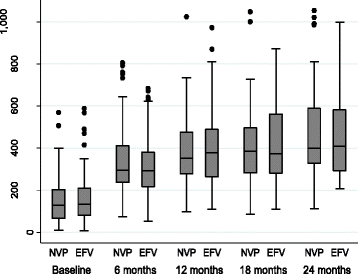
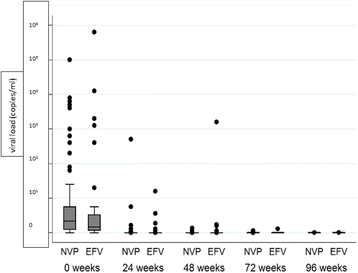
Results: A total of 284 patients (mean age 36.7 ± 8.1 years) were randomized in a 1:1 ratio to receive either Nevirapine (n = 144)- or Efavirenz (n = 140)-based ART after a median ATT-ART gap of 27 days. The median CD4 count was 105 cells/μl, with a median viral load of 820,200 copies/μl and no significant difference between the groups. Composite unfavourable outcomes were reported in 49 patients in the Nevirapine group and 51 patients in the Efavirenz group (35.3% vs. 36.9%; hazard ratio, 0.95, 95% confidence interval (CI), 0.63,1.43, adjusted). There was no difference in successful TB treatment outcome between the groups (71.5% vs. 65.6%, 95% CI -3.8,17.9, adjusted). The results were similar, showing no difference between the groups in the two centres of the study after adjusting for disease stage.
Conclusions: Composite unfavourable outcome in HIV-TB co-infected patients who were ART-naïve showed no statistically significant difference in the Nevirapine or Efavirenz groups.. Therefore, Nevirapine-based ART is a reasonable alternative to Efavirenz in resource-limited settings. However, multi-centric studies with larger sample sizes are required to confirm these findings.
Trial registration: NCT01805258 (Retrospectively registered on March 6, 2013) Date of registration: March 2013.
Keywords: ART naïve; ATT; Antiretroviral; Efavirenz; Nevirapine; Tuberculosis.
Conflict of interest statement
Ethics approval and consent to participate
This study has been approved by the Institute Ethics Committee, All-India Institute of Medical Sciences, New Delhi, India. Before the start of the study, all participants provided written informed consent. Participants were informed that they had the right to discontinue their participation or withdraw from the study at any time.
Consent for publication
Consent was taken from both the ethics committee and the subjects at the time of enrolment.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Low A, Gavriilidis G, Larke N, B-Lajoie MR, Drouin O, Stover J, Muhe L, Easterbrook P. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2016;62(12):1595–1603. doi: 10.1093/cid/ciw125. - DOI - PMC - PubMed
-
- World Health Organization: Global tuberculosis report 2016. Geneva: WHO; 2016. Available: http://www.who.int/tb/publications/global_report/gtbr2016_executive_summ...
-
- National AIDS Control Organisation India . Department of AIDSControl, Ministry of Health & Family Welfare: Annual Report 2015–16. New Delhi: National AIDS control organization; 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

