Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial
- PMID: 29228935
- PMCID: PMC5725796
- DOI: 10.1186/s12890-017-0535-y
Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial
Abstract
Background: Systemic morphine has evidence to support its use for reducing breathlessness in patients with severe chronic obstructive pulmonary disease (COPD). The effectiveness of the nebulized route, however, has not yet been confirmed. Recent studies have shown that opioid receptors are localized within epithelium of human trachea and large bronchi, a target site for a dosimetric nebulizer. The aim of this study was to compare any clinical or statistical differences in breathlessness intensity between nebulized 2.0% morphine and 0,9% NaCl in patients with very severe COPD.
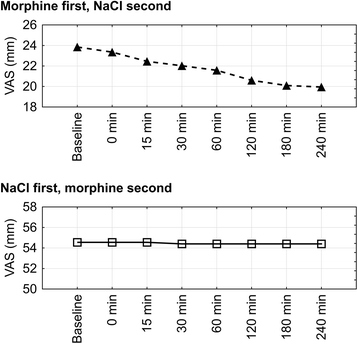
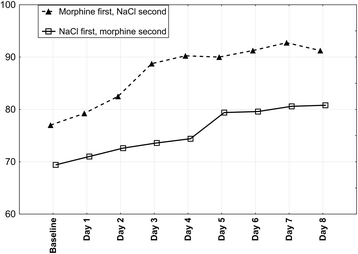
Methods: The study was a double-blind, controlled, cross-over trial. Participants received morphine or NaCl during two 4-day periods. Sequence of periods was randomized. The primary outcome measure was reduction of breathlessness intensity now by ≥20 mm using a 100 mm visual analogue scale (VAS) at baseline, 15, 30, 60, 120, 180 and 240 min after daily administration, during normal activities.
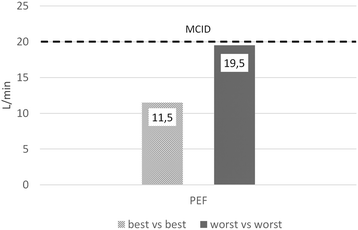
Results: Ten of 11 patients included completed the study protocol. All patients experienced clinically and statistically significant (p < 0.0001) breathlessness reduction during morphine nebulization. Mean VAS changes for morphine and 0.9% NaCl periods were 25.4 mm (standard deviation (SD): 9.0; median: 23,0; range: 14.0 to 41,5; confidence interval (CI): 95%) and 6.3 mm (SD: 7.8; median: 6.8; range: -11,5 to 19,5; CI: 95%), respectively. No treatment emergent adverse effects were noted.
Discussion: Our study showed superiority of dosimetrically administered nebulized morphine compared to NaCl in reducing breathlessness. This may have been achieved through morphine's direct action on receptors in large airways, although a systemic effect from absorption through the lungs cannot be excluded.
Trial registration: Retrospectively registered (07.03.2017), ISRCTN14865597.
Conflict of interest statement
Ethics approval and consent to participate
Study protocol was approved by the Independent Bioethics Committee for Research of Medical University of Gdansk (NKBBN/269/2012). Study participants provided written, informed consent.
Consent for publication
Not applicable.
Competing interests
PJ, MK, TB, ID-K, PS, DCC, EJ declare no conflict of interest. ZP is the manufacturer of the inhalation device used in the study (PNEUMONEB®). Author did not participate in collection and analysis of data.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Opioids in patients with COPD and refractory dyspnea: literature review and design of a multicenter double blind study of low dosed morphine and fentanyl (MoreFoRCOPD).BMC Pulm Med. 2021 Sep 10;21(1):289. doi: 10.1186/s12890-021-01647-8. BMC Pulm Med. 2021. PMID: 34507574 Free PMC article. Review.
-
Effect of Sustained-Release Morphine for Refractory Breathlessness in Chronic Obstructive Pulmonary Disease on Health Status: A Randomized Clinical Trial.JAMA Intern Med. 2020 Oct 1;180(10):1306-1314. doi: 10.1001/jamainternmed.2020.3134. JAMA Intern Med. 2020. PMID: 32804188 Free PMC article. Clinical Trial.
-
Effect of Regular, Low-Dose, Extended-release Morphine on Chronic Breathlessness in Chronic Obstructive Pulmonary Disease: The BEAMS Randomized Clinical Trial.JAMA. 2022 Nov 22;328(20):2022-2032. doi: 10.1001/jama.2022.20206. JAMA. 2022. PMID: 36413230 Free PMC article. Clinical Trial.
-
Harms of Morphine for Chronic Breathlessness in Relation to Dose, Duration and Titration Phase.J Pain Symptom Manage. 2025 Jun;69(6):581-588.e2. doi: 10.1016/j.jpainsymman.2025.02.020. Epub 2025 Feb 25. J Pain Symptom Manage. 2025. PMID: 40015533 Clinical Trial.
-
Nebulized morphine for relief of dyspnea due to chronic lung disease.Ann Pharmacother. 2005 Jun;39(6):1088-92. doi: 10.1345/aph.1E328. Epub 2005 Apr 19. Ann Pharmacother. 2005. PMID: 15840735 Review.
Cited by
-
Opioids in Treatment of Refractory Dyspnea in Chronic Obstructive Pulmonary Disease: Yes, No or Maybe.J Pers Med. 2024 Mar 19;14(3):318. doi: 10.3390/jpm14030318. J Pers Med. 2024. PMID: 38541060 Free PMC article. Review.
-
Opioids in patients with COPD and refractory dyspnea: literature review and design of a multicenter double blind study of low dosed morphine and fentanyl (MoreFoRCOPD).BMC Pulm Med. 2021 Sep 10;21(1):289. doi: 10.1186/s12890-021-01647-8. BMC Pulm Med. 2021. PMID: 34507574 Free PMC article. Review.
-
A case report of an 18-year-old receiving nebulized lidocaine for treatment of COVID-19 cough.Heart Lung. 2023 Jan-Feb;57:140-143. doi: 10.1016/j.hrtlng.2022.09.009. Epub 2022 Sep 22. Heart Lung. 2023. PMID: 36201924 Free PMC article.
-
Efficacy and Safety of Topical Morphine: A Narrative Review.Pharmaceutics. 2022 Jul 19;14(7):1499. doi: 10.3390/pharmaceutics14071499. Pharmaceutics. 2022. PMID: 35890392 Free PMC article. Review.
References
-
- Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

