Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus
- PMID: 29228970
- PMCID: PMC5725948
- DOI: 10.1186/s12987-017-0083-0
Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus
Abstract
Background: Neuroinflammation has been implicated in the pathophysiology of post-hemorrhagic hydrocephalus (PHH) of prematurity, but no comprehensive analysis of signaling molecules has been performed using human cerebrospinal fluid (CSF).
Methods: Lumbar CSF levels of key cytokines (IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, TGF-β1, IFN-γ) and chemokines (XCL-1, CCL-2, CCL-3, CCL-19, CXCL-10, CXCL-11, CXCL-12) were measured using conventional and multiplexed Enzyme-linked Immunosorbent Assays and compared between preterm infants with PHH and those with no known neurological injury. The relationships between individual biomarker levels and specific CSF cell counts were examined.
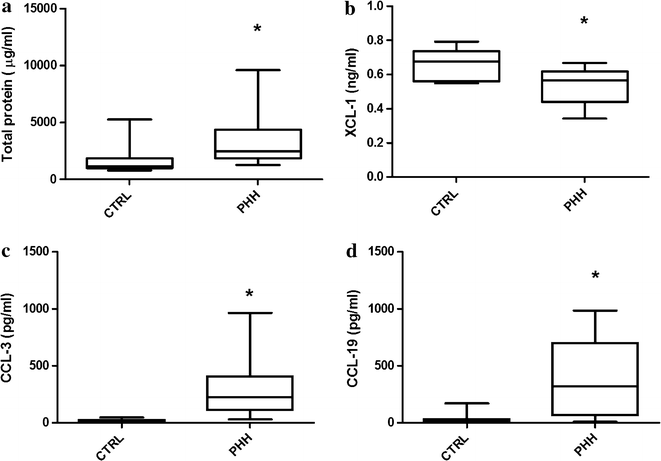
Results: Total protein (TP) CSF levels were elevated in the PHH subjects compared to controls. CSF levels of IL-1α, IL-4, IL-6, IL-12, TNF-α, CCL-3, CCL-19, and CXCL-10 were significantly increased in PHH whereas XCL-1 was significantly decreased in PHH. When normalizing by TP, IL-1α, IL-1β, IL-10, IL-12, CCL-3, and CCL-19 levels were significantly elevated compared to controls, while XCL-1 levels remained significantly decreased. Among those with significantly different levels in both absolute and normalized levels, only absolute CCL-19 levels showed a significant correlation with CSF nucleated cells, neutrophils, and lymphocytes. IL-1β and CXCL-10 also were correlated with total cell count, nucleated cells, red blood cells, and neutrophils.
Conclusions: Neuroinflammation is likely to be an important process in the pathophysiology of PHH. To our knowledge, this is the first study to investigate CSF levels of chemokines in PHH as well as the only one to show XCL-1 selectively decreased in a diseased state. Additionally, CCL-19 was the only analyte studied that showed significant differences between groups and had significant correlation with cell count analysis. The selectivity of CCL-19 and XCL-1 should be further investigated. Future studies will further delineate the role of these cytokines and chemokines in PHH.
Keywords: CSF; Cerebrospinal fluid; Chemokines; Cytokines; Hydrocephalus; Post-hemorrhagic; Prematurity; Preterm.
Figures
Similar articles
-
Biochemical profile of human infant cerebrospinal fluid in intraventricular hemorrhage and post-hemorrhagic hydrocephalus of prematurity.Fluids Barriers CNS. 2021 Dec 24;18(1):62. doi: 10.1186/s12987-021-00295-8. Fluids Barriers CNS. 2021. PMID: 34952604 Free PMC article.
-
Cerebrospinal fluid NCAM-1 concentration is associated with neurodevelopmental outcome in post-hemorrhagic hydrocephalus of prematurity.PLoS One. 2021 Mar 10;16(3):e0247749. doi: 10.1371/journal.pone.0247749. eCollection 2021. PLoS One. 2021. PMID: 33690655 Free PMC article.
-
Lumbar Cerebrospinal Fluid Biomarkers of Posthemorrhagic Hydrocephalus of Prematurity: Amyloid Precursor Protein, Soluble Amyloid Precursor Protein α, and L1 Cell Adhesion Molecule.Neurosurgery. 2017 Jan 1;80(1):82-90. doi: 10.1227/NEU.0000000000001415. Neurosurgery. 2017. PMID: 27571524 Free PMC article.
-
Neuroinflammatory pathways and potential therapeutic targets in neonatal post-hemorrhagic hydrocephalus.Pediatr Res. 2025 Mar;97(4):1345-1357. doi: 10.1038/s41390-024-03733-z. Epub 2024 Dec 26. Pediatr Res. 2025. PMID: 39725707 Review.
-
An Integrative Review of Cytokine/Chemokine Predictors of Neurodevelopment in Preterm Infants.Biol Res Nurs. 2019 Jul;21(4):366-376. doi: 10.1177/1099800419852766. Epub 2019 May 29. Biol Res Nurs. 2019. PMID: 31142128 Free PMC article. Review.
Cited by
-
Impaired neurogenesis with reactive astrocytosis in the hippocampus in a porcine model of acquired hydrocephalus.Exp Neurol. 2023 May;363:114354. doi: 10.1016/j.expneurol.2023.114354. Epub 2023 Feb 21. Exp Neurol. 2023. PMID: 36822393 Free PMC article.
-
Immune activation during Paenibacillus brain infection in African infants with frequent cytomegalovirus co-infection.iScience. 2021 Mar 23;24(4):102351. doi: 10.1016/j.isci.2021.102351. eCollection 2021 Apr 23. iScience. 2021. PMID: 33912816 Free PMC article.
-
Association of inflammatory cytokines expression in cerebrospinal fluid with the severity and prognosis of spontaneous intracerebral hemorrhage.BMC Neurol. 2024 Jan 2;24(1):7. doi: 10.1186/s12883-023-03487-x. BMC Neurol. 2024. PMID: 38167007 Free PMC article.
-
Elevated Pro-Inflammatory Cell-Free MicroRNA Levels in Cerebrospinal Fluid of Premature Infants after Intraventricular Hemorrhage.Int J Mol Sci. 2020 Sep 19;21(18):6870. doi: 10.3390/ijms21186870. Int J Mol Sci. 2020. PMID: 32961661 Free PMC article. Clinical Trial.
-
Paediatric hydrocephalus.Nat Rev Dis Primers. 2024 May 16;10(1):35. doi: 10.1038/s41572-024-00519-9. Nat Rev Dis Primers. 2024. PMID: 38755194 Free PMC article. Review.
References
-
- Lekic T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman RE, Altay O, Tang J, Zhang JH. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Exp Neurol. 2012;236:69–78. doi: 10.1016/j.expneurol.2012.04.003. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

