Alpha-synuclein oligomer-selective antibodies reduce intracellular accumulation and mitochondrial impairment in alpha-synuclein exposed astrocytes
- PMID: 29228971
- PMCID: PMC5725978
- DOI: 10.1186/s12974-017-1018-z
Alpha-synuclein oligomer-selective antibodies reduce intracellular accumulation and mitochondrial impairment in alpha-synuclein exposed astrocytes
Abstract
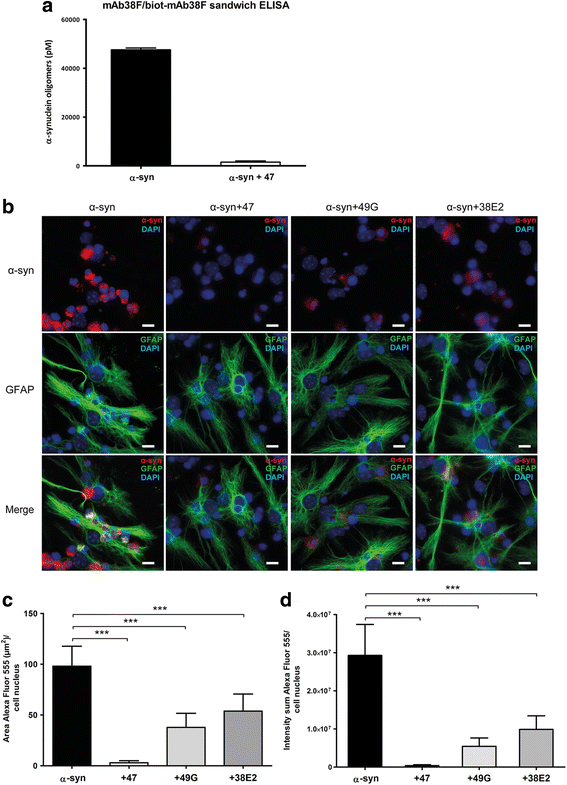
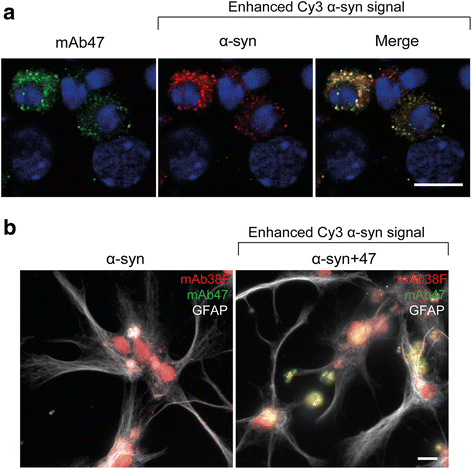
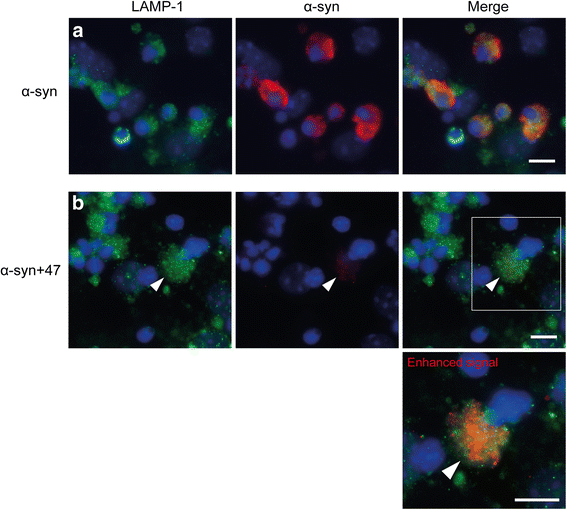
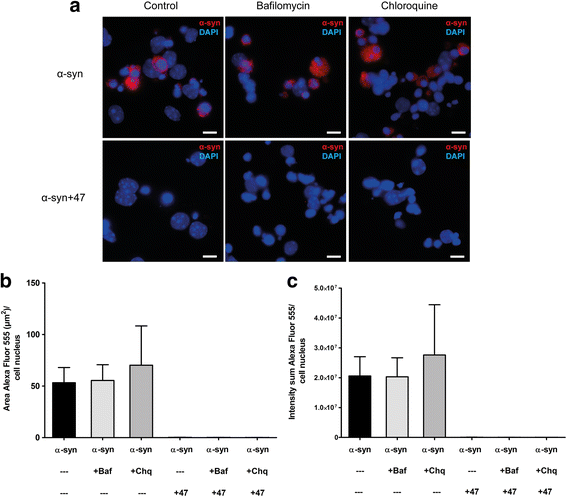
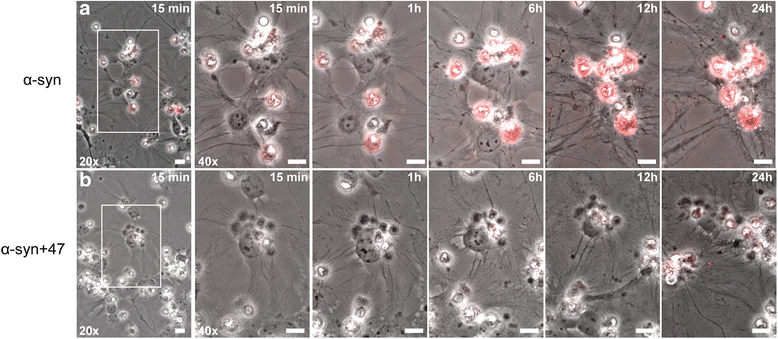
Background: Due to its neurotoxic properties, oligomeric alpha-synuclein (α-syn) has been suggested as an attractive target for passive immunization against Parkinson's disease (PD). In mouse models of PD, antibody treatment has been shown to lower the levels of pathogenic α-syn species, including oligomers, although the mechanisms of action remain unknown. We have previously shown that astrocytes rapidly engulf α-syn oligomers that are intracellularly stored, rather than degraded, resulting in impaired mitochondria.
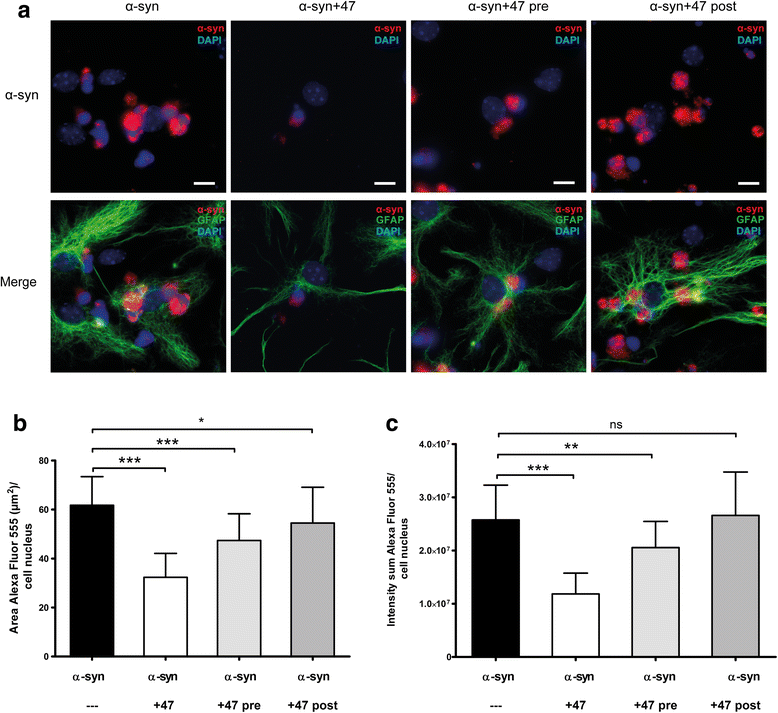
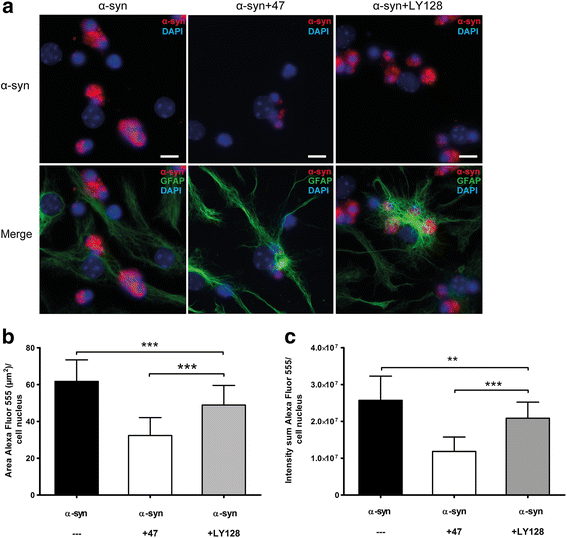
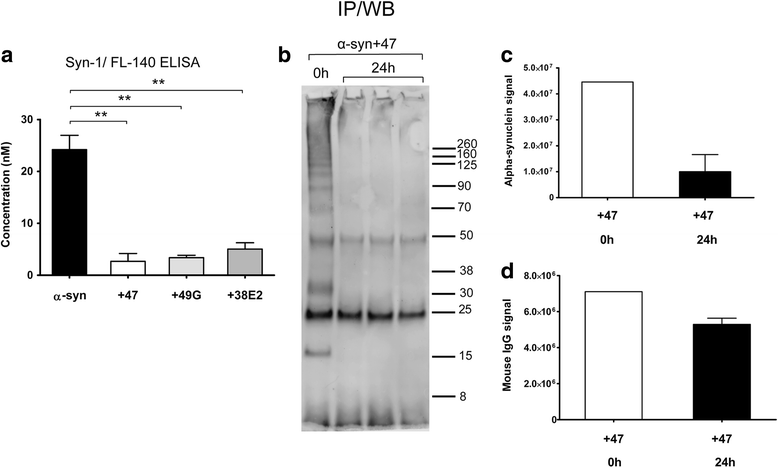
Methods: The aim of the present study was to investigate if the accumulation of α-syn in astrocytes can be affected by α-syn oligomer-selective antibodies. Co-cultures of astrocytes, neurons, and oligodendrocytes were derived from embryonic mouse cortex and exposed to α-syn oligomers or oligomers pre-incubated with oligomer-selective antibodies.
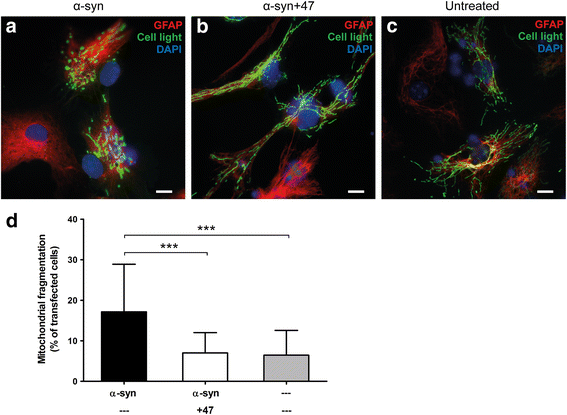
Results: In the presence of antibodies, the astrocytes displayed an increased clearance of the exogenously added α-syn, and consequently, the α-syn accumulation in the culture was markedly reduced. Moreover, the addition of antibodies rescued the astrocytes from the oligomer-induced mitochondrial impairment.
Conclusions: Our results demonstrate that oligomer-selective antibodies can prevent α-syn accumulation and mitochondrial dysfunction in cultured astrocytes.
Keywords: Antibodies; Astrocytes; Lysosomal degradation; Mitochondria; Parkinson’s disease; α-synuclein oligomers.
Conflict of interest statement
Ethics approval
All experiments involving animals were performed at Uppsala University, Sweden. The experiments were approved by the Uppsala County Animal Ethics Board (ethical permit number: C75/13, valid 2013-06-28 to 2018-06-28), following the rules and regulations of the Swedish Animal Welfare Agency, in compliance with the European Communities Council Directive of 22 September 2010 (2010/63/EU).
Consent for publication
Not applicable.
Competing interests
EN is employed by BioArctic Neuroscience AB. LL is co-founder of BioArctic AB and stock owner. This does not alter to the Journal of Neuroinflammation policies on sharing data and materials. None of the authors have any financial relationship with the organizations that sponsored the research. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
Grants and funding
- 2015/Parkinson Research Foundation (SE)
- 2015-02671/Vetenskapsrådet
- 931/16/Parkinsonfonden
- 932/16/Parkinsonfonden
- AF-646541/Alzheimerfonden
- AF-647381/Alzheimerfonden
- mC27/h16/Åhlén-stiftelsen
- mA17/h15/Åhlén-stiftelsen
- M-2017-0555/Petrus och Augusta Hedlunds Stiftelse
- AD1/Uppsala Berzelii Center
- 2017/Demensförbundet
- 2016/Lennart och Kristina Kalén
- MMW 2011.0109/Marianne and Marcus Wallenberg Foundation
- 2016/The U4 Ageing Brain Network
- 2017/Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

