Asiatic acid attenuates methamphetamine-induced neuroinflammation and neurotoxicity through blocking of NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway
- PMID: 29228978
- PMCID: PMC5725763
- DOI: 10.1186/s12974-017-1009-0
Asiatic acid attenuates methamphetamine-induced neuroinflammation and neurotoxicity through blocking of NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway
Abstract
Background: Methamphetamine (METH) is a commonly abused drug that may result in neurotoxic effects. Recent studies have suggested that involvement of neuroinflammatory processes in brain dysfunction is induced by misuse of this drug. However, the mechanism underlying METH-induced inflammation and neurotoxicity in neurons is still unclear. In this study, we investigated whether asiatic acid (AA) effected METH-mediated neuroinflammation and neurotoxicity in dopaminergic neuronal cells. And we further determined whether the effect involved in the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and signal transducer and activator of transcription (STAT)3 and extracellular signal-regulated kinase (ERK) pathway.
Methods: We used the human dopaminergic neuroblastoma SH-SY5Y cell line, murine microglial BV2 cell line, and primary culture of rat embryo mesencephalic neurons. Pro-inflammatory cytokine production was monitored by ELISA and RT/real-time PCR. The cell cycle distribution and mitochondrial membrane integrity was analyzed by flow cytometry. We used immunoblotting, DNA-binding activity, and immunofluorescence staining to analyze the effect of AA on activation of the NF-κB, STAT3, MAPK-ERK, and apoptosis signaling pathways.
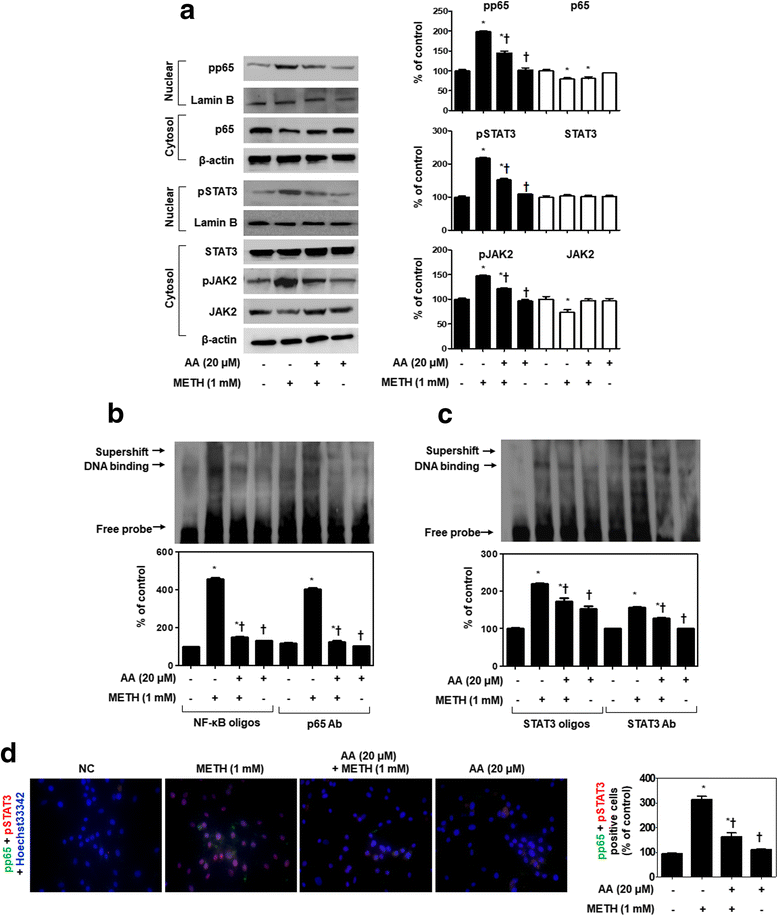
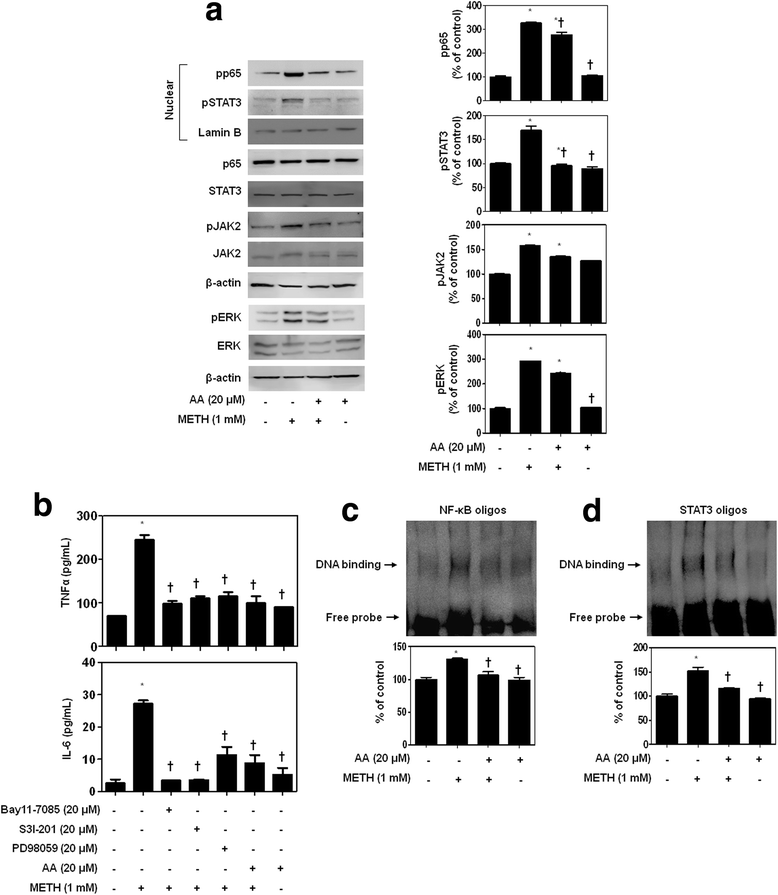
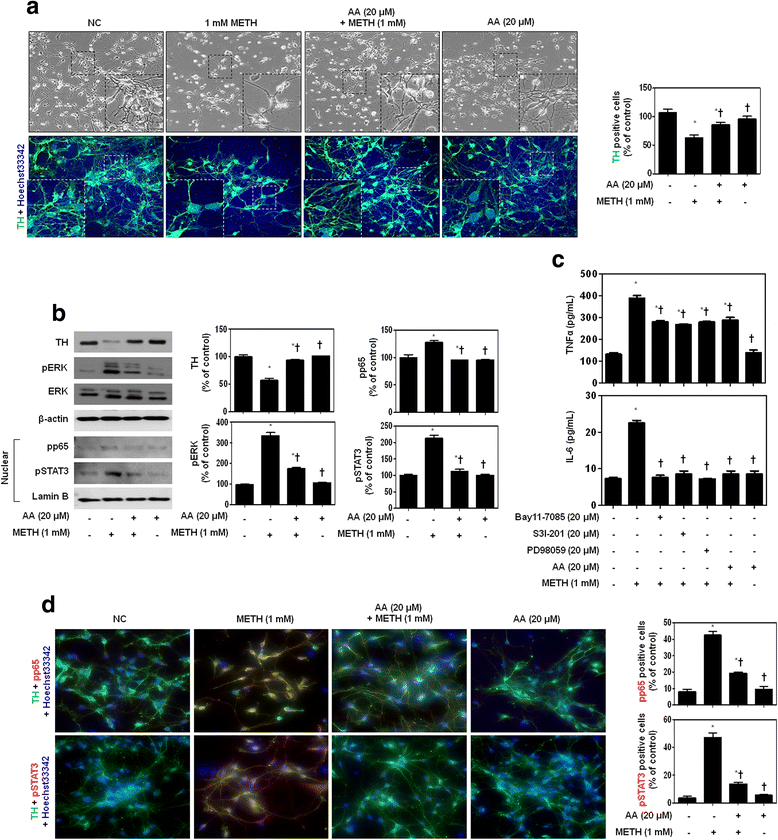
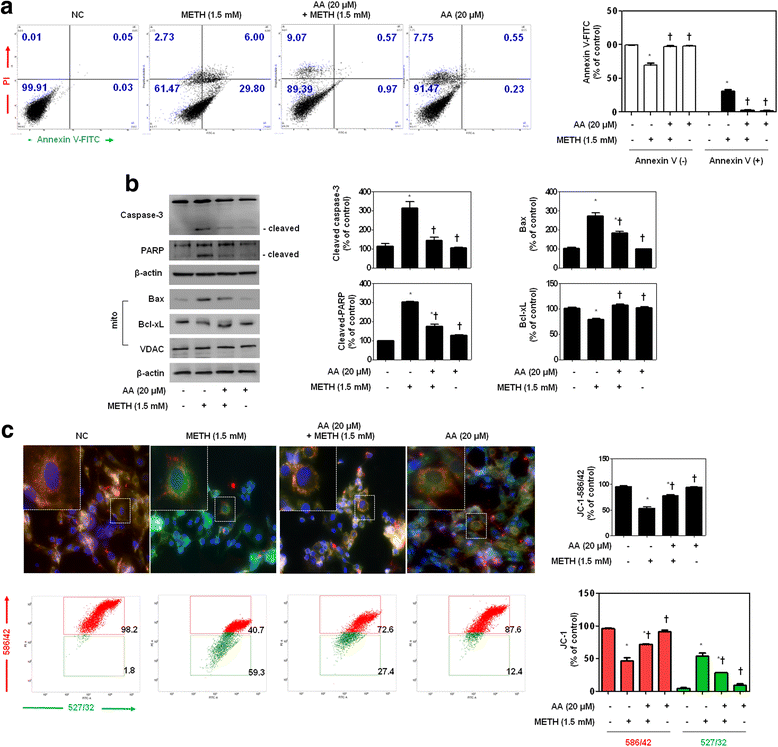
Results: METH induced TNF receptor (TNFR) expression and led to morphological changes of cells. Additionally, this drug increased pro-inflammatory cytokine (TNFα and IL-6) expression. AA significantly suppressed METH-induced TNFR expression in concentration dependent. Increased secretion of TNFα and IL-6 was inhibited in METH-stimulated neuronal cells by AA administration. AA showed significant protection against METH-induced translocation of NF-κB/STAT3 and ERK phosphorylation. AA inhibited METH-induced proteolytic fragmentation of caspase-3 and PARP. The pro-apoptotic protein Bax was significantly decreased, while the anti-apoptotic protein Bcl-xL was increased by AA treatment in METH-stimulated cells. A similar protective effect of AA on mitochondrial membrane integrity was also confirmed by flow cytometry and immunofluorescence staining.
Conclusions: Based on the literatures and our findings, AA is a promising candidate for an anti-neurotoxic agent, and it can potentially be used for the prevention and treatment of various neurological disorders.
Keywords: Asiatic acid; ERK; Methamphetamine; NF-κB; Neuroinflammation; Neurotoxicity; STAT3.
Conflict of interest statement
Ethics approval
Experimental procedures were approved by the Institutional Animal Care and Use Committee of the Keimyung University in accordance with the criteria outlined in the Institutional Guidelines for Animal Research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Lupenone Protects Neuroblastoma SH-SY5y Cells Against Methamphetamine-Induced Apoptotic Cell Death via PI3K/Akt/mTOR Signaling Pathway.Int J Mol Sci. 2020 Feb 27;21(5):1617. doi: 10.3390/ijms21051617. Int J Mol Sci. 2020. PMID: 32120831 Free PMC article.
-
Involvement of C/EBPβ-related signaling pathway in methamphetamine-induced neuronal autophagy and apoptosis.Toxicol Lett. 2019 Sep 15;312:11-21. doi: 10.1016/j.toxlet.2019.05.003. Epub 2019 May 3. Toxicol Lett. 2019. PMID: 31059759
-
CDK5-mediated tau accumulation triggers methamphetamine-induced neuronal apoptosis via endoplasmic reticulum-associated degradation pathway.Toxicol Lett. 2018 Aug;292:97-107. doi: 10.1016/j.toxlet.2018.04.027. Epub 2018 Apr 26. Toxicol Lett. 2018. PMID: 29705343
-
Possible mechanism for the neuroprotective effects of L-carnitine on methamphetamine-evoked neurotoxicity.Ann N Y Acad Sci. 2003 May;993:197-207; discussion 287-8. doi: 10.1111/j.1749-6632.2003.tb07530.x. Ann N Y Acad Sci. 2003. PMID: 12853314 Review.
-
Methamphetamine neurotoxicity, microglia, and neuroinflammation.J Neuroinflammation. 2018 Dec 12;15(1):341. doi: 10.1186/s12974-018-1385-0. J Neuroinflammation. 2018. PMID: 30541633 Free PMC article. Review.
Cited by
-
Activation of RARα Receptor Attenuates Neuroinflammation After SAH via Promoting M1-to-M2 Phenotypic Polarization of Microglia and Regulating Mafb/Msr1/PI3K-Akt/NF-κB Pathway.Front Immunol. 2022 Feb 14;13:839796. doi: 10.3389/fimmu.2022.839796. eCollection 2022. Front Immunol. 2022. PMID: 35237277 Free PMC article.
-
Lupenone Protects Neuroblastoma SH-SY5y Cells Against Methamphetamine-Induced Apoptotic Cell Death via PI3K/Akt/mTOR Signaling Pathway.Int J Mol Sci. 2020 Feb 27;21(5):1617. doi: 10.3390/ijms21051617. Int J Mol Sci. 2020. PMID: 32120831 Free PMC article.
-
K284-6111 alleviates memory impairment and neuroinflammation in Tg2576 mice by inhibition of Chitinase-3-like 1 regulating ERK-dependent PTX3 pathway.J Neuroinflammation. 2020 Nov 22;17(1):350. doi: 10.1186/s12974-020-02022-w. J Neuroinflammation. 2020. PMID: 33222690 Free PMC article.
-
S100A1 expression is increased in spinal cord injury and promotes inflammation, oxidative stress and apoptosis of PC12 cells induced by LPS via ERK signaling.Mol Med Rep. 2023 Feb;27(2):30. doi: 10.3892/mmr.2022.12917. Epub 2022 Dec 16. Mol Med Rep. 2023. PMID: 36524376 Free PMC article.
-
The Role of Oxidative Stress and Inflammation in the Pathogenesis and Treatment of Vascular Dementia.Cells. 2025 Apr 17;14(8):609. doi: 10.3390/cells14080609. Cells. 2025. PMID: 40277934 Free PMC article. Review.
References
-
- Goncalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO, Silva AP. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur J Neurosci. 2010;31:315–326. doi: 10.1111/j.1460-9568.2009.07059.x. - DOI - PubMed
-
- Shin EJ, Shin SW, Nguyen TT, Park DH, Wie MB, Jang CG, Nah SY, Yang BW, Ko SK, Nabeshima T, Kim HC. Ginsenoside Re rescues methamphetamine-induced oxidative damage, mitochondrial dysfunction, microglial activation, and dopaminergic degeneration by inhibiting the protein kinase Cdelta gene. Mol Neurobiol. 2014;49:1400–1421. doi: 10.1007/s12035-013-8617-1. - DOI - PubMed
-
- Nguyen XK, Lee J, Shin EJ, Dang DK, Jeong JH, Nguyen TT, Nam Y, Cho HJ, Lee JC, Park DH, et al. Liposomal melatonin rescues methamphetamine-elicited mitochondrial burdens, pro-apoptosis, and dopaminergic degeneration through the inhibition PKCdelta gene. J Pineal Res. 2015;58:86–106. doi: 10.1111/jpi.12195. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

