Clinical role of breast MRI now and going forward
- PMID: 29229179
- PMCID: PMC6788454
- DOI: 10.1016/j.crad.2017.10.021
Clinical role of breast MRI now and going forward
Abstract
Magnetic resonance imaging (MRI) is a well-established method in breast imaging, with manifold clinical applications, including the non-invasive differentiation between benign and malignant breast lesions, preoperative staging, detection of scar versus recurrence, implant assessment, and the evaluation of high-risk patients. At present, dynamic contrast-enhanced MRI is the most sensitive imaging technique for breast cancer diagnosis, and provides excellent morphological and to some extent also functional information. To compensate for the limited functional information, and to increase the specificity of MRI while preserving its sensitivity, additional functional parameters such as diffusion-weighted imaging and apparent diffusion coefficient mapping, and MR spectroscopic imaging have been investigated and implemented into the clinical routine. Several additional MRI parameters to capture breast cancer biology are still under investigation. MRI at high and ultra-high field strength and advances in hard- and software may also further improve this imaging technique. This article will review the current clinical role of breast MRI, including multiparametric MRI and abbreviated protocols, and provide an outlook on the future of this technique. In addition, the predictive and prognostic value of MRI as well as the evolving field of radiogenomics will be discussed.
Copyright © 2017 The Royal College of Radiologists. Published by Elsevier Ltd. All rights reserved.
Figures


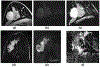
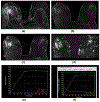
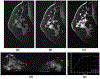




References
-
- Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;46(8): 1296–1316. - PubMed
-
- Pinker K, Bogner W, Baltzer P, et al. Clinical application of bilateral high temporal and spatial resolution dynamic contrast-enhanced MR imaging of the breast at 7 T. Eur Radiol. 2014;24(4):913–920. - PubMed
-
- Pinker-Domenig K, Bogner W, Gruber S, et al. High resolution MRI of the breast at 3 T: which BI-RADS(R) descriptors are most strongly associated with the diagnosis of breast cancer? Eur Radiol. 2012;22(2):322–330. - PubMed
-
- Morris EA. Diagnostic breast MR imaging: current status and future directions. Radiol Clin North Am. 2007;45(5):863–880, vii. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

