Neural control of choroidal blood flow
- PMID: 29229444
- PMCID: PMC5971129
- DOI: 10.1016/j.preteyeres.2017.12.001
Neural control of choroidal blood flow
Abstract
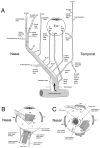
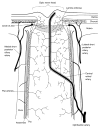
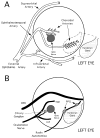
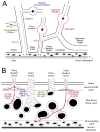
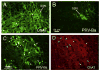
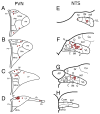
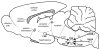
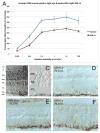
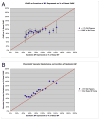
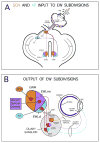
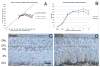
The choroid is richly innervated by parasympathetic, sympathetic and trigeminal sensory nerve fibers that regulate choroidal blood flow in birds and mammals, and presumably other vertebrate classes as well. The parasympathetic innervation has been shown to vasodilate and increase choroidal blood flow, the sympathetic input has been shown to vasoconstrict and decrease choroidal blood flow, and the sensory input has been shown to both convey pain and thermal information centrally and act locally to vasodilate and increase choroidal blood flow. As the choroid lies behind the retina and cannot respond readily to retinal metabolic signals, its innervation is important for adjustments in flow required by either retinal activity, by fluctuations in the systemic blood pressure driving choroidal perfusion, and possibly by retinal temperature. The former two appear to be mediated by the sympathetic and parasympathetic nervous systems, via central circuits responsive to retinal activity and systemic blood pressure, but adjustments for ocular perfusion pressure also appear to be influenced by local autoregulatory myogenic mechanisms. Adaptive choroidal responses to temperature may be mediated by trigeminal sensory fibers. Impairments in the neural control of choroidal blood flow occur with aging, and various ocular or systemic diseases such as glaucoma, age-related macular degeneration (AMD), hypertension, and diabetes, and may contribute to retinal pathology and dysfunction in these conditions, or in the case of AMD be a precondition. The present manuscript reviews findings in birds and mammals that contribute to the above-summarized understanding of the roles of the autonomic and sensory innervation of the choroid in controlling choroidal blood flow, and in the importance of such regulation for maintaining retinal health.
Keywords: Choroidal blood flow; Ciliary ganglion; Ocular blood flow; Parasympathetic; Pterygopalatine ganglion; Superior cervical ganglion; Sympathetic; Uvea.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures











References
-
- Abe S, Karita K, Izumi H, Tamai M. Increased and decreased choroidal blood flow elicited by cervical sympathetic nerve stimulation in the cat. Jpn J Physiol. 1995;45:347–353. - PubMed
-
- Agassandian K, Fazan VPS, Cassell MD, Lin LH, Talman WT. Direct projections from the cardiovascular nucleus tractus solitarii to pontine preganglionic parasympathetic neurons: a link to cerebrovascular regulation. J Comp Neurol. 2002;452:242–254. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

