Optical coherence tomography angiography
- PMID: 29229445
- PMCID: PMC6404988
- DOI: 10.1016/j.preteyeres.2017.11.003
Optical coherence tomography angiography
Abstract
Optical coherence tomography (OCT) was one of the biggest advances in ophthalmic imaging. Building on that platform, OCT angiography (OCTA) provides depth resolved images of blood flow in the retina and choroid with levels of detail far exceeding that obtained with older forms of imaging. This new modality is challenging because of the need for new equipment and processing techniques, current limitations of imaging capability, and rapid advancements in both imaging and in our understanding of the imaging and applicable pathophysiology of the retina and choroid. These factors lead to a steep learning curve, even for those with a working understanding dye-based ocular angiography. All for a method of imaging that is a little more than 10 years old. This review begins with a historical account of the development of OCTA, and the methods used in OCTA, including signal processing, image generation, and display techniques. This forms the basis to understand what OCTA images show as well as how image artifacts arise. The anatomy and imaging of specific vascular layers of the eye are reviewed. The integration of OCTA in multimodal imaging in the evaluation of retinal vascular occlusive diseases, diabetic retinopathy, uveitis, inherited diseases, age-related macular degeneration, and disorders of the optic nerve is presented. OCTA is an exciting, disruptive technology. Its use is rapidly expanding in clinical practice as well as for research into the pathophysiology of diseases of the posterior pole.
Keywords: Multimodal imaging; Optical coherence tomography; Optical coherence tomography angiography.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures













































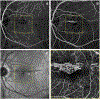














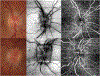


References
-
- Adhi M, Bonini Filho MA, Louzada RN, Kuehlewein L, Talisa E, Baumal CR, Witkin AJ, Sadda SR, Sarraf D, Reichel E, 2016. Retinal capillary network and foveal avascular zone in eyes with vein occlusion and fellow eyes analyzed with optical coherence tomography AngiographyRetinal capillary network and FAZ in RVO with OCTA. Invest. Ophthalmol. Vis. Sci 57, - PubMed
-
- Aessopos A, Voskaridou E, Kavouklis E, Vassilopoulos G, Rombos Y, Gavriel L, Loukopoulos D, 1994. Angioid streaks in sickle-thalassemia. Am. J. Ophthalmol 117, 589–592. - PubMed
-
- Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, Gentile RC, Hsiao YS, Zhou Q, Ko T, Rosen RB, 2015. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 35, 2353–2363. - PubMed
-
- Aiello LM, 2003. Perspectives on diabetic retinopathy. Am. J. Ophthalmol 136, 122–135. - PubMed
-
- Akagi T, Iida Y, Nakanishi H, Terada N, Morooka S, Yamada H, Hasegawa T, Yokota S, Yoshikawa M, Yoshimura N, 2016. Microvascular density in glaucomatous eyes with hemifield visual field defects: an optical coherence tomography angiography study. Am. J. Ophthalmol 168, 237–249. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

