Recent advances in pre-exposure prophylaxis for HIV
- PMID: 29229609
- PMCID: PMC6020995
- DOI: 10.1136/bmj.j5011
Recent advances in pre-exposure prophylaxis for HIV
Abstract
Although pre-exposure prophylaxis (PrEP)-the use of antiretroviral drugs by non-infected people to prevent the acquisition of HIV-is a promising preventive option, important public health questions remain. Daily oral emtricitabine (FTC)-tenofovir disoproxil fumarate (TDF) is highly efficacious in preventing the acquisition of HIV in people at risk as a result of a range of different types of sexual exposure. There is good evidence of efficacy in women and men, and when men who have sex with men use event based dosing. Studies have been conducted in several countries and epidemics. Because adherence to this treatment varies greatly there are questions about its public health benefit. Oral FTC-TDF is extremely safe, with minimal impact on kidney, bone, or pregnancy outcomes, and there is no evidence that its effectiveness has been reduced by risk compensation during open label and programmatic follow-up. It is too early to assess the impact of this treatment on the incidence of sexually transmitted infections (STIs) at a population level. Many challenges remain. Access to pre-exposure prophylaxis is limited and disparities exist, including those governed by race and sex. Different pricing and access models need to be explored to avoid further widening inequalities. The optimal combination prevention program needs to be defined, and this will depend on local epidemiology, service provision, and cost effectiveness. This review updates the evidence base for pre-exposure prophylaxis regarding its effectiveness, safety, and risk compensation.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: SMcC was the principal investigator and MD the trial physician in the PROUD study, which was funded in part by Gilead, which provided the drugs for free. MD, NF, and SMcC were on the writing group of the UK national pre-exposure prophylaxis guidelines. RG was the principal investigator of the iPrEx study.
Figures
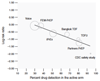



References
-
- Cambiano V, Miners A, Dunn D, McCormack S, Gill N, Nardone A, et al. O1 Is pre-exposure prophylaxis for hiv prevention cost-effective in men who have sex with men who engage in condomless sex in the uk? Sex Transm Infect. 2015 Sep 23;91 [Internet]. Available from: http://sti.bmj.com/content/91/Suppl_1/A1.1.abstract.
-
- Paltiel AD, Freedberg KA, Scott CA, Schackman BR, Losina E, Wang B, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(6):806–15. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
