Opposing Actions of Fibroblast and Cardiomyocyte Smad3 Signaling in the Infarcted Myocardium
- PMID: 29229611
- PMCID: PMC5812838
- DOI: 10.1161/CIRCULATIONAHA.117.029622
Opposing Actions of Fibroblast and Cardiomyocyte Smad3 Signaling in the Infarcted Myocardium
Abstract
Background: Transforming growth factor-βs regulate a wide range of cellular responses by activating Smad-dependent and Smad-independent cascades. In the infarcted heart, Smad3 signaling is activated in both cardiomyocytes and interstitial cells. We hypothesized that cell-specific actions of Smad3 regulate repair and remodeling in the infarcted myocardium.
Methods: To dissect cell-specific Smad3 actions in myocardial infarction, we generated mice with Smad3 loss in activated fibroblasts or cardiomyocytes. Cardiac function was assessed after reperfused or nonreperfused infarction using echocardiography. The effects of cell-specific Smad3 loss on the infarcted heart were studied using histological studies, assessment of protein, and gene expression levels. In vitro, we studied Smad-dependent and Smad-independent actions in isolated cardiac fibroblasts.
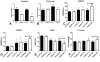
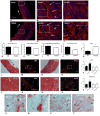
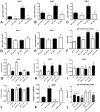
Results: Mice with fibroblast-specific Smad3 loss had accentuated adverse remodeling after reperfused infarction and exhibited an increased incidence of late rupture after nonreperfused infarction. The consequences of fibroblast-specific Smad3 loss were not a result of effects on acute infarct size but were associated with unrestrained fibroblast proliferation, impaired scar remodeling, reduced fibroblast-derived collagen synthesis, and perturbed alignment of myofibroblast arrays in the infarct. Polarized light microscopy in Sirius red-stained sections demonstrated that the changes in fibroblast morphology were associated with perturbed organization of the collagenous matrix in the infarcted area. In contrast, α-smooth muscle actin expression by infarct myofibroblasts was not affected by Smad3 loss. Smad3 critically regulated fibroblast function, activating integrin-mediated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-2 (NOX-2) expression. Smad3 loss in cardiomyocytes attenuated remodeling and dysfunction after infarction. Cardiomyocyte-specific Smad3 loss did not affect acute infarct size but was associated with attenuated cardiomyocyte apoptosis in the remodeling myocardium, accompanied by decreased myocardial NOX-2 levels, reduced nitrosative stress, and lower matrix metalloproteinase-2 expression.
Conclusions: In healing myocardial infarction, myofibroblast- and cardiomyocyte-specific activation of Smad3 has contrasting functional outcomes that may involve activation of an integrin/reactive oxygen axis.
Keywords: SMAD; cardiomyocyte; fibroblast; heart failure; remodeling.
© 2017 American Heart Association, Inc.
Figures



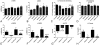

References
-
- Deten A, Holzl A, Leicht M, Barth W, Zimmer HG. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol. 2001;33:1191–1207. - PubMed
-
- Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999;31:667–678. - PubMed
-
- Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–2138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

