Metabolic Vulnerabilities of Prostate Cancer: Diagnostic and Therapeutic Opportunities
- PMID: 29229664
- PMCID: PMC6169980
- DOI: 10.1101/cshperspect.a030569
Metabolic Vulnerabilities of Prostate Cancer: Diagnostic and Therapeutic Opportunities
Abstract
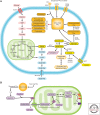
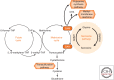
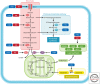
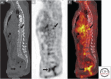
Cancer cells hijack metabolic pathways to support bioenergetics and biosynthetic requirements for their uncontrolled growth. Thus, cancer can be considered as a metabolic disease. In this review, we discuss the main metabolic features of prostate cancer with a particular focus on the link between oncogene-directed cancer metabolic regulation, metabolism rewiring, and epigenetic regulation. The potential of using metabolic profiling as a means to predict disease behavior and to identify novel therapeutic targets and new diagnostic markers will be addressed as well as the current challenges in metabolomics analyses. Finally, diagnostic and prognostic metabolic imaging approaches, including positron emission tomography, mass spectrometry, nuclear magnetic resonance, and their translational applications, will be discussed. Here, we emphasize how targeting metabolic vulnerabilities in prostate cancer may pave the way for novel personalized diagnostic and therapeutic interventions.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
Application of metabolomics to prostate cancer.Urol Oncol. 2011 Sep-Oct;29(5):572-81. doi: 10.1016/j.urolonc.2011.08.002. Urol Oncol. 2011. PMID: 21930089 Free PMC article. Review.
-
Identification of the perturbed metabolic pathways associating with prostate cancer cells and anticancer affects of obacunone.J Proteomics. 2019 Aug 30;206:103447. doi: 10.1016/j.jprot.2019.103447. Epub 2019 Jul 19. J Proteomics. 2019. PMID: 31326558
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Application of proteomic technologies for prostate cancer detection, prognosis, and tailored therapy.Crit Rev Clin Lab Sci. 2010 May-Jun;47(3):125-38. doi: 10.3109/10408363.2010.503558. Epub 2010 Sep 21. Crit Rev Clin Lab Sci. 2010. PMID: 20858067 Review.
-
The applications of metabolomics in the molecular diagnostics of cancer.Expert Rev Mol Diagn. 2019 Sep;19(9):785-793. doi: 10.1080/14737159.2019.1656530. Epub 2019 Aug 21. Expert Rev Mol Diagn. 2019. PMID: 31414918 Review.
Cited by
-
Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention.Adv Drug Deliv Rev. 2020;159:245-293. doi: 10.1016/j.addr.2020.07.013. Epub 2020 Jul 23. Adv Drug Deliv Rev. 2020. PMID: 32711004 Free PMC article. Review.
-
Lipogenic effects of androgen signaling in normal and malignant prostate.Asian J Urol. 2020 Jul;7(3):258-270. doi: 10.1016/j.ajur.2019.12.003. Epub 2019 Dec 10. Asian J Urol. 2020. PMID: 32742926 Free PMC article. Review.
-
Identifying a Ferroptosis-Related Gene Signature for Predicting Biochemical Recurrence of Prostate Cancer.Front Cell Dev Biol. 2021 Oct 29;9:666025. doi: 10.3389/fcell.2021.666025. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34778244 Free PMC article.
-
Role of Lipids and Lipid Metabolism in Prostate Cancer Progression and the Tumor's Immune Environment.Cancers (Basel). 2022 Sep 1;14(17):4293. doi: 10.3390/cancers14174293. Cancers (Basel). 2022. PMID: 36077824 Free PMC article. Review.
-
Metabolically regulated lineages in prostate cancer.Nat Cell Biol. 2023 Dec;25(12):1726-1728. doi: 10.1038/s41556-023-01298-3. Nat Cell Biol. 2023. PMID: 38049603 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
