Expression of Toll-Like Receptor 2 by Dendritic Cells Is Essential for the DnaJ-ΔA146Ply-Mediated Th1 Immune Response against Streptococcus pneumoniae
- PMID: 29229733
- PMCID: PMC5820941
- DOI: 10.1128/IAI.00651-17
Expression of Toll-Like Receptor 2 by Dendritic Cells Is Essential for the DnaJ-ΔA146Ply-Mediated Th1 Immune Response against Streptococcus pneumoniae
Abstract
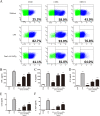
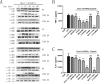
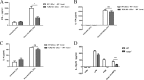
The fusion protein DnaJ-ΔA146Ply could induce cross-protective immunity against pneumococcal infection via mucosal and subcutaneous immunization in mice in the absence of additional adjuvants. DnaJ and Ply are both Toll-like receptor 4 (TLR4) but not TLR2 ligands. However, we found that TLR2-/- mice immunized subcutaneously with DnaJ-ΔA146Ply showed significantly lower survival rates and higher bacterial loads in nasal washes than did wild-type (WT) mice after being challenged with pneumococcal strain D39 or 19F. The gamma interferon (IFN-γ) level in splenocytes decreased in TLR2-/- mice, indicating that Th1 immunity elicited by DnaJ-ΔA146Ply was impaired in these mice. We explored the mechanism of protective immunity conferred by DnaJ-ΔA146Ply and the role of TLR2 in this process. DnaJ-ΔA146Ply effectively promoted dendritic cell (DC) maturation via TLR4 but not the TLR2 signaling pathway. In a DnaJ-ΔA146Ply-treated DC and naive CD4+ T cell coculture system, the deficiency of TLR2 in DCs resulted in a significant decline of IFN-γ production and Th1 subset differentiation. The same effect was observed in adoptive-transfer experiments. In addition, TLR2-/- DCs showed remarkably lower levels of the Th1-polarizing cytokine IL-12p70 than did WT DCs, suggesting that TLR2 was indispensable for DnaJ-ΔA146Ply-induced IL-12 production and Th1 proliferation. Thus, our findings illustrate that dendritic cell expression of TLR2 is essential for optimal Th1 immune response against pneumococci in mice immunized subcutaneously with DnaJ-ΔA146Ply.
Keywords: DnaJ-ΔA146Ply; Th1 immunity; Toll-like receptor 2; dendritic cells; protein vaccine.
Copyright © 2018 American Society for Microbiology.
Figures


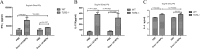


Similar articles
-
IL-4 plays an essential role in DnaJ-ΔA146Ply-mediated immunoprotection against Streptococcus pneumoniae in mice.Mol Immunol. 2022 Mar;143:105-113. doi: 10.1016/j.molimm.2022.01.010. Epub 2022 Feb 1. Mol Immunol. 2022. PMID: 35114487
-
Pneumococcal DnaJ modulates dendritic cell-mediated Th1 and Th17 immune responses through Toll-like receptor 4 signaling pathway.Immunobiology. 2017 Feb;222(2):384-393. doi: 10.1016/j.imbio.2016.08.013. Epub 2016 Aug 31. Immunobiology. 2017. PMID: 27594384
-
Mucosal immunization with recombinant fusion protein DnaJ-ΔA146Ply enhances cross-protective immunity against Streptococcus pneumoniae infection in mice via interleukin 17A.Infect Immun. 2014 Apr;82(4):1666-75. doi: 10.1128/IAI.01391-13. Epub 2014 Feb 3. Infect Immun. 2014. PMID: 24491576 Free PMC article.
-
Subcutaneous Immunization with Fusion Protein DnaJ-ΔA146Ply without Additional Adjuvants Induces both Humoral and Cellular Immunity against Pneumococcal Infection Partially Depending on TLR4.Front Immunol. 2017 Jun 12;8:686. doi: 10.3389/fimmu.2017.00686. eCollection 2017. Front Immunol. 2017. PMID: 28659923 Free PMC article.
-
Reassessing the usual suspects causing a commotion in the blood (and vessels?).Cancer Biol Ther. 2004 Jan;3(1):124-5. doi: 10.4161/cbt.3.1.685. Epub 2004 Jan 9. Cancer Biol Ther. 2004. PMID: 14739778 Review. No abstract available.
Cited by
-
The Recombinant Profilin from Free-Living Amoebae Induced Allergic Immune Responses via TLR2.J Inflamm Res. 2024 May 13;17:2915-2925. doi: 10.2147/JIR.S450866. eCollection 2024. J Inflamm Res. 2024. PMID: 38764493 Free PMC article.
-
Role of toll-like receptors in post-COVID-19 associated neurodegenerative disorders?Front Med (Lausanne). 2025 Mar 26;12:1458281. doi: 10.3389/fmed.2025.1458281. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40206484 Free PMC article. Review.
-
Protein-Based Adjuvants for Vaccines as Immunomodulators of the Innate and Adaptive Immune Response: Current Knowledge, Challenges, and Future Opportunities.Pharmaceutics. 2022 Aug 11;14(8):1671. doi: 10.3390/pharmaceutics14081671. Pharmaceutics. 2022. PMID: 36015297 Free PMC article. Review.
-
Bacterial Protein Toll-Like-Receptor Agonists: A Novel Perspective on Vaccine Adjuvants.Front Immunol. 2019 May 29;10:1144. doi: 10.3389/fimmu.2019.01144. eCollection 2019. Front Immunol. 2019. PMID: 31191528 Free PMC article. Review.
-
Toll-like Receptor 2 in Autoimmune Inflammation.Immune Netw. 2021 Jun 25;21(3):e18. doi: 10.4110/in.2021.21.e18. eCollection 2021 Jun. Immune Netw. 2021. PMID: 34277108 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

