The Superantigen Toxic Shock Syndrome Toxin 1 Alters Human Aortic Endothelial Cell Function
- PMID: 29229737
- PMCID: PMC5820935
- DOI: 10.1128/IAI.00848-17
The Superantigen Toxic Shock Syndrome Toxin 1 Alters Human Aortic Endothelial Cell Function
Abstract
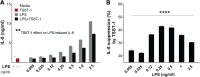
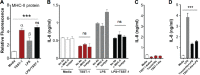
Staphylococcus aureus infective endocarditis (IE) is a fast-progressing and tissue-destructive infection of the cardiac endothelium. The superantigens (SAgs) toxic shock syndrome toxin 1 (TSST-1), staphylococcal enterotoxin C (SEC), and the toxins encoded by the enterotoxin gene cluster (egc) play a novel and essential role in the etiology of S. aureus IE. Recent studies indicate that SAgs act at the infection site to cause tissue pathology and promote vegetation growth. The underlying mechanism of SAg involvement has not been clearly defined. In SAg-mediated responses, immune cell priming is considered a primary triggering event leading to endothelial cell activation and altered function. Utilizing immortalized human aortic endothelial cells (iHAECs), we demonstrated that TSST-1 directly activates iHAECs, as documented by upregulation of vascular and intercellular adhesion molecules (VCAM-1 and ICAM-1). TSST-1-mediated activation results in increased monolayer permeability and defects in vascular reendothelialization. Yet stimulation of iHAECs with TSST-1 fails to induce interleukin-8 (IL-8) and IL-6 production. Furthermore, simultaneous stimulation of iHAECs with TSST-1 and lipopolysaccharide (LPS) inhibits LPS-mediated IL-8 and IL-6 secretion, even after pretreatment with either of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and IL-1β. IL-8 suppression is not mediated by TSST-1 binding to its canonical receptor major histocompatibility complex class II (MHC-II), supporting current evidence for a nonhematopoietic interacting site on SAgs. Together, the data suggest that TSST-1 differentially regulates cell-bound and secreted markers of endothelial cell activation that may result in dysregulated innate immune responses during S. aureus IE. Endothelial changes resulting from the action of SAgs can therefore directly contribute to the aggressive nature of S. aureus IE and development of life-threatening complications.
Keywords: TSST-1; aortic endothelial cell dysfunction; human endothelial cells; infective endocarditis; superantigen.
Copyright © 2018 Kulhankova et al.
Figures






Similar articles
-
Epithelial proinflammatory response and curcumin-mediated protection from staphylococcal toxic shock syndrome toxin-1.PLoS One. 2012;7(3):e32813. doi: 10.1371/journal.pone.0032813. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22431984 Free PMC article.
-
Induction of tumor necrosis factor alpha and interleukin-8 gene expression in bronchial epithelial cells by toxic shock syndrome toxin 1.Infect Immun. 2000 Jan;68(1):120-4. doi: 10.1128/IAI.68.1.120-124.2000. Infect Immun. 2000. PMID: 10603377 Free PMC article.
-
MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: Defining a novel mechanism of superantigen-induced immunopathology and immunosuppression.PLoS Biol. 2017 Jun 20;15(6):e2001930. doi: 10.1371/journal.pbio.2001930. eCollection 2017 Jun. PLoS Biol. 2017. PMID: 28632753 Free PMC article.
-
Staphylococcal toxic shock syndrome: superantigen-mediated enhancement of endotoxin shock and adaptive immune suppression.Immunol Res. 2014 Aug;59(1-3):182-7. doi: 10.1007/s12026-014-8538-8. Immunol Res. 2014. PMID: 24816557 Review.
-
Allergy-A New Role for T Cell Superantigens of Staphylococcus aureus?Toxins (Basel). 2020 Mar 12;12(3):176. doi: 10.3390/toxins12030176. Toxins (Basel). 2020. PMID: 32178378 Free PMC article. Review.
Cited by
-
ϕSa3mw Prophage as a Molecular Regulatory Switch of Staphylococcus aureus β-Toxin Production.J Bacteriol. 2019 Jun 21;201(14):e00766-18. doi: 10.1128/JB.00766-18. Print 2019 Jul 15. J Bacteriol. 2019. PMID: 30962356 Free PMC article.
-
Staphylococcus aureus bloodstream infections: pathogenesis and regulatory mechanisms.Curr Opin Microbiol. 2020 Feb;53:51-60. doi: 10.1016/j.mib.2020.02.005. Epub 2020 Mar 12. Curr Opin Microbiol. 2020. PMID: 32172183 Free PMC article. Review.
-
Aetiological Significance of Infectious Stimuli in Kawasaki Disease.Front Pediatr. 2019 Jun 28;7:244. doi: 10.3389/fped.2019.00244. eCollection 2019. Front Pediatr. 2019. PMID: 31316950 Free PMC article. Review.
-
Virulence Factor Genes and Antimicrobial Susceptibility of Staphylococcus aureus Strains Isolated from Blood and Chronic Wounds.Toxins (Basel). 2021 Jul 14;13(7):491. doi: 10.3390/toxins13070491. Toxins (Basel). 2021. PMID: 34357963 Free PMC article.
-
Toxic Shock Syndrome Toxin-1 (TSST-1) in Staphylococcus aureus: Prevalence, Molecular Mechanisms, and Public Health Implications.Toxins (Basel). 2025 Jun 24;17(7):323. doi: 10.3390/toxins17070323. Toxins (Basel). 2025. PMID: 40711134 Free PMC article. Review.
References
-
- Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH, International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473. doi:10.1001/archinternmed.2008.603. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

