Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes
- PMID: 29229807
- PMCID: PMC5748171
- DOI: 10.1073/pnas.1707876115
Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes
Abstract
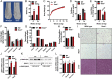
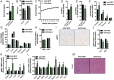
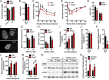
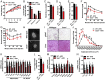
Sclerostin has traditionally been thought of as a local inhibitor of bone acquisition that antagonizes the profound osteoanabolic capacity of activated Wnt/β-catenin signaling, but serum sclerostin levels in humans exhibit a correlation with impairments in several metabolic parameters. These data, together with the increased production of sclerostin in mouse models of type 2 diabetes, suggest an endocrine function. To determine whether sclerostin contributes to the coordination of whole-body metabolism, we examined body composition, glucose homeostasis, and fatty acid metabolism in Sost-/- mice as well as mice that overproduce sclerostin as a result of adeno-associated virus expression from the liver. Here, we show that in addition to dramatic increases in bone volume, Sost-/- mice exhibit a reduction in adipose tissue accumulation in association with increased insulin sensitivity. Sclerostin overproduction results in the opposite metabolic phenotype due to adipocyte hypertrophy. Additionally, Sost-/- mice and those administered a sclerostin-neutralizing antibody are resistant to obesogenic diet-induced disturbances in metabolism. This effect appears to be the result of sclerostin's effects on Wnt signaling and metabolism in white adipose tissue. Since adipocytes do not produce sclerostin, these findings suggest an unexplored endocrine function for sclerostin that facilitates communication between the skeleton and adipose tissue.
Keywords: Wnt; adipose; bone; metabolism; sclerostin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Lrp4 expression by adipocytes and osteoblasts differentially impacts sclerostin's endocrine effects on body composition and glucose metabolism.J Biol Chem. 2019 Apr 26;294(17):6899-6911. doi: 10.1074/jbc.RA118.006769. Epub 2019 Mar 6. J Biol Chem. 2019. PMID: 30842262 Free PMC article.
-
Bone-derived sclerostin and Wnt/β-catenin signaling regulate PDGFRα+ adipoprogenitor cell differentiation.FASEB J. 2021 Nov;35(11):e21957. doi: 10.1096/fj.202100691R. FASEB J. 2021. PMID: 34606641 Free PMC article.
-
Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots.J Bone Miner Res. 2017 Feb;32(2):373-384. doi: 10.1002/jbmr.3001. Epub 2017 Jan 5. J Bone Miner Res. 2017. PMID: 27653320 Free PMC article.
-
Sclerostin as a new target of diabetes-induced osteoporosis.Front Endocrinol (Lausanne). 2024 Dec 10;15:1491066. doi: 10.3389/fendo.2024.1491066. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39720253 Free PMC article. Review.
-
Sclerostin: From Molecule to Clinical Biomarker.Int J Mol Sci. 2022 Apr 26;23(9):4751. doi: 10.3390/ijms23094751. Int J Mol Sci. 2022. PMID: 35563144 Free PMC article. Review.
Cited by
-
The rs1634330 Polymorphisms in the SOST Gene Are Associated with Body Composition in Chinese Nuclear Families with Male Offspring.Int J Endocrinol. 2021 May 8;2021:6698822. doi: 10.1155/2021/6698822. eCollection 2021. Int J Endocrinol. 2021. PMID: 34054948 Free PMC article.
-
High-Throughput Screening of Mouse Gene Knockouts Identifies Established and Novel High Body Fat Phenotypes.Diabetes Metab Syndr Obes. 2021 Aug 28;14:3753-3785. doi: 10.2147/DMSO.S322083. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34483672 Free PMC article.
-
Emerging insights into the comparative effectiveness of anabolic therapies for osteoporosis.Nat Rev Endocrinol. 2021 Jan;17(1):31-46. doi: 10.1038/s41574-020-00426-5. Epub 2020 Nov 4. Nat Rev Endocrinol. 2021. PMID: 33149262 Review.
-
Cytokines, Adipokines, and Bone Markers at Rest and in Response to Plyometric Exercise in Obese vs Normal Weight Adolescent Females.Front Endocrinol (Lausanne). 2020 Dec 11;11:531926. doi: 10.3389/fendo.2020.531926. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33362710 Free PMC article.
-
Low Bone Turnover Due to Hypothyroidism or Anti-Resorptive Treatment Does Not Affect Whole-Body Glucose Homeostasis in Male Mice.J Pers Med. 2022 Sep 6;12(9):1462. doi: 10.3390/jpm12091462. J Pers Med. 2022. PMID: 36143246 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

