Disease onset in X-linked dystonia-parkinsonism correlates with expansion of a hexameric repeat within an SVA retrotransposon in TAF1
- PMID: 29229810
- PMCID: PMC5754783
- DOI: 10.1073/pnas.1712526114
Disease onset in X-linked dystonia-parkinsonism correlates with expansion of a hexameric repeat within an SVA retrotransposon in TAF1
Erratum in
-
Correction for Cristopher Bragg et al., Disease onset in X-linked dystonia-parkinsonism correlates with expansion of a hexameric repeat within an SVA retrotransposon in TAF1.Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):6277. doi: 10.1073/pnas.2003190117. Epub 2020 Mar 9. Proc Natl Acad Sci U S A. 2020. PMID: 32152096 Free PMC article. No abstract available.
Abstract
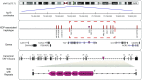
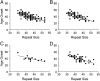
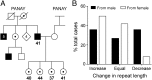
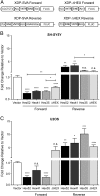
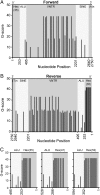
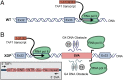
X-linked dystonia-parkinsonism (XDP) is a neurodegenerative disease associated with an antisense insertion of a SINE-VNTR-Alu (SVA)-type retrotransposon within an intron of TAF1 This unique insertion coincides with six additional noncoding sequence changes in TAF1, the gene that encodes TATA-binding protein-associated factor-1, which appear to be inherited together as an identical haplotype in all reported cases. Here we examined the sequence of this SVA in XDP patients (n = 140) and detected polymorphic variation in the length of a hexanucleotide repeat domain, (CCCTCT)n The number of repeats in these cases ranged from 35 to 52 and showed a highly significant inverse correlation with age at disease onset. Because other SVAs exhibit intrinsic promoter activity that depends in part on the hexameric domain, we assayed the transcriptional regulatory effects of varying hexameric lengths found in the unique XDP SVA retrotransposon using luciferase reporter constructs. When inserted sense or antisense to the luciferase reading frame, the XDP variants repressed or enhanced transcription, respectively, to an extent that appeared to vary with length of the hexamer. Further in silico analysis of this SVA sequence revealed multiple motifs predicted to form G-quadruplexes, with the greatest potential detected for the hexameric repeat domain. These data directly link sequence variation within the XDP-specific SVA sequence to phenotypic variability in clinical disease manifestation and provide insight into potential mechanisms by which this intronic retroelement may induce transcriptional interference in TAF1 expression.
Keywords: DYT3; Parkinson’s disease; TAF1; XDP; dystonia.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lee LV, Pascasio FM, Fuentes FD, Viterbo GH. Torsion dystonia in Panay, Philippines. Adv Neurol. 1976;14:137–151. - PubMed
-
- Lee LV, Kupke KG, Caballar-Gonzaga F, Hebron-Ortiz M, Müller U. The phenotype of the X-linked dystonia-parkinsonism syndrome. An assessment of 42 cases in the Philippines. Medicine (Baltimore) 1991;70:179–187. - PubMed
-
- Lee LV, et al. The unique phenomenology of sex-linked dystonia parkinsonism (XDP, DYT3, “Lubag”) Int J Neurosci. 2011;121:3–11. - PubMed
-
- Lee LV, et al. The natural history of sex-linked recessive dystonia parkinsonism of Panay, Philippines (XDP) Parkinsonism Relat Disord. 2002;9:29–38. - PubMed
-
- Evidente VG, et al. Phenomenology of “Lubag” or X-linked dystonia-parkinsonism. Mov Disord. 2002;17:1271–1277. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

