FKBP12 contributes to α-synuclein toxicity by regulating the calcineurin-dependent phosphoproteome
- PMID: 29229832
- PMCID: PMC5748183
- DOI: 10.1073/pnas.1711926115
FKBP12 contributes to α-synuclein toxicity by regulating the calcineurin-dependent phosphoproteome
Abstract
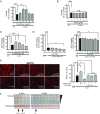
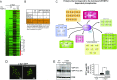
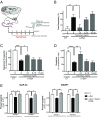
Calcineurin is an essential Ca2+-dependent phosphatase. Increased calcineurin activity is associated with α-synuclein (α-syn) toxicity, a protein implicated in Parkinson's Disease (PD) and other neurodegenerative diseases. Calcineurin can be inhibited with Tacrolimus through the recruitment and inhibition of the 12-kDa cis-trans proline isomerase FK506-binding protein (FKBP12). Whether calcineurin/FKBP12 represents a native physiologically relevant assembly that occurs in the absence of pharmacological perturbation has remained elusive. We leveraged α-syn as a model to interrogate whether FKBP12 plays a role in regulating calcineurin activity in the absence of Tacrolimus. We show that FKBP12 profoundly affects the calcineurin-dependent phosphoproteome, promoting the dephosphorylation of a subset of proteins that contributes to α-syn toxicity. Using a rat model of PD, partial elimination of the functional interaction between FKBP12 and calcineurin, with low doses of the Food and Drug Administration (FDA)-approved compound Tacrolimus, blocks calcineurin's activity toward those proteins and protects against the toxic hallmarks of α-syn pathology. Thus, FKBP12 can endogenously regulate calcineurin activity with therapeutic implications for the treatment of PD.
Keywords: FKBP12; Parkinson’s Disease; Tacrolimus; calcineurin; α-synuclein.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rusnak F, Mertz P. Calcineurin: Form and function. Physiol Rev. 2000;80:1483–1521. - PubMed
-
- Martí MJ, Tolosa E, Campdelacreu J. Clinical overview of the synucleinopathies. Mov Disord. 2003;18(Suppl 6):S21–S27. - PubMed
-
- Hurwitz MY, Putkey JA, Klee CB, Means AR. Domain II of calmodulin is involved in activation of calcineurin. FEBS Lett. 1988;238:82–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

