A Multicenter Phase III Study to Evaluate the Efficacy and Safety of Hepabulin, a New Hepatitis B Immunoglobulin, in Liver Transplantation Recipients with Hepatitis B
- PMID: 29229898
- PMCID: PMC6248297
- DOI: 10.12659/aot.905898
A Multicenter Phase III Study to Evaluate the Efficacy and Safety of Hepabulin, a New Hepatitis B Immunoglobulin, in Liver Transplantation Recipients with Hepatitis B
Abstract
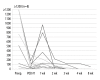
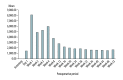
BACKGROUND This study was performed to evaluate the effects and stability of the new hepatitis B immunoglobulin (HBIG), Hepabulin, in patients undergoing liver transplantation for hepatitis B. MATERIAL AND METHODS A total of 87 patients undergoing liver transplantation for hepatitis B-related liver disease were enrolled in this multicenter, phase III, open-label, single-arm study. Seventy (80.5%) of the 87 enrolled patients completed the study during the 52-week study period. Hepabulin (10,000 units) was intravenously injected intraoperatively, daily for 1 week, weekly for 1 month, and then once per month. Hepabulin was used as monotherapy without antiviral agents. Hepatitis B recurrence was defined as conversion from negativity for surface antigen after HBIG administration to positivity. RESULTS There were no cases of hepatitis B recurrence during the 52-week observation period. A total of 876 adverse events (AEs) that occurred during the study period were observed in 83 (95.4%) of 87 patients, and serious AEs were seen in 119 cases in 44 (50.6%) of the 87 patients. None of the AEs showed a relationship with this drug. Hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) rapidly disappeared within 1 week after HBIG administration, but hepatitis B virus (HBV) DNA persisted for up to 8 weeks after surgery, which was related to HBV viral load. Hepatitis B surface antibody (HBsAb) was correlated with HBIG (Hepabulin) dose. CONCLUSIONS The new HBIG, Hepabulin, was shown to be safe and effective in preventing the recurrence of HBV after liver transplantation.
Figures

Similar articles
-
Recurrence-free long-term survival after liver transplantation for hepatitis B using interferon-alpha pretransplant and hepatitis B immune globulin posttransplant.Ann Surg. 1997 Sep;226(3):356-65; discussion 365-8. doi: 10.1097/00000658-199709000-00015. Ann Surg. 1997. PMID: 9339942 Free PMC article. Clinical Trial.
-
An efficacy and cost-effectiveness analysis of combination hepatitis B immune globulin and lamivudine to prevent recurrent hepatitis B after orthotopic liver transplantation compared with hepatitis B immune globulin monotherapy.Liver Transpl. 2000 Nov;6(6):741-8. doi: 10.1053/jlts.2000.18702. Liver Transpl. 2000. PMID: 11084061
-
Minimization of anti-hepatitis B surface antigen immunoglobulins for prophylaxis of hepatitis B viral recurrence in the first month after liver transplantation: the meaning of HBsAg quantitative level at the time of transplant.Transplant Proc. 2014 Sep;46(7):2308-11. doi: 10.1016/j.transproceed.2014.07.043. Transplant Proc. 2014. PMID: 25242775
-
Efficacy and effectiveness of anti-HBV therapy with early withdrawal of HBIG prophylaxis to prevent HBV recurrence following liver transplantation.Expert Opin Biol Ther. 2015 May;15(5):665-77. doi: 10.1517/14712598.2015.1025045. Expert Opin Biol Ther. 2015. PMID: 25865452 Review.
-
Hepatitis B immunoglobulin for prevention of hepatitis B virus infection and recurrence after liver transplantation.Expert Rev Clin Immunol. 2011 Jul;7(4):429-36. doi: 10.1586/eci.11.30. Expert Rev Clin Immunol. 2011. PMID: 21790285 Review.
Cited by
-
Determination of Hepatitis B Immunoglobulin Infusion Interval Using Pharmacokinetic Half-life Simulation for Posttransplant Hepatitis B Prophylaxis.J Korean Med Sci. 2019 Oct 7;34(38):e251. doi: 10.3346/jkms.2019.34.e251. J Korean Med Sci. 2019. PMID: 31583871 Free PMC article.
-
Assessment of patient safety and the efficiency of facility utilization following simplified ultra-rapid intravenous infusion of hepatitis B immunoglobulin in a high-volume liver transplantation center.Ann Hepatobiliary Pancreat Surg. 2019 May;23(2):128-132. doi: 10.14701/ahbps.2019.23.2.128. Epub 2019 May 31. Ann Hepatobiliary Pancreat Surg. 2019. PMID: 31225413 Free PMC article.
-
Hepatitis B Prophylaxis after Liver Transplantation in Korea: Analysis of the KOTRY Database.J Korean Med Sci. 2020 Feb 17;35(6):e36. doi: 10.3346/jkms.2020.35.e36. J Korean Med Sci. 2020. PMID: 32056398 Free PMC article.
References
-
- Korea Centers for Disease Control and Prevention. The Third Korea National Health and Nutrition Examination Survey (KNHANES III), 2005. Seoul: Korea Centers for Disease Control and Prevention; 2006. p. 68.
-
- Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842–47. - PubMed
-
- Kim WR, Poterucha JJ, Kremers WK, et al. Outcome of liver transplantation for hepatitis B in the United States. Liver Transpl. 2004;10:968–74. - PubMed
-
- Kasraianfard A, Watt KD, Lindberg L, et al. HBIG remains significant in the era of new potent nucleoside analogues for prophylaxis against hepatitis B recurrence after liver transplantation. Int Rev Immunol. 2016;35:312–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

