Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue
- PMID: 29229920
- PMCID: PMC5725567
- DOI: 10.1038/s41598-017-17411-0
Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue
Abstract
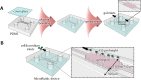
Neurovascular unit (NVU) is a basic unit in the brain, including neurons, glial cells, blood vessels and extracellular matrix. This concept implies the importance of a three-dimensional (3D) culture model including these cell types for investigating brain functions. However, little is known about the construction of an in vitro 3D NVU model. In the present study, we aimed at constructing 3D neurovascular tissues by combining in vitro neurogenesis and angiogenesis models using a microfluidic platform, which is a critical step toward the NVU construction in vitro. Three gel conditions, which were fibrin gel, fibrin-Matrigel mixed gel and fibrin-hyaluronan mixed gel, were investigated to optimize the gel components in terms of neurogenesis and angiogenesis. First, fibrin-Matrigel mixed gel was found to promote neural stem cell (NSC) differentiation into neurons and neurite extension. In particular, 3D neural networks were constructed in 2-8 mg/ml fibrin-Matrigel mixed gel. Second, we found that capillary-like structures were also formed in the fibrin-Matrigel mixed gel by coculturing brain microvascular endothelial cells (BMECs) and human mesenchymal stem cells (MSCs). Finally, we combined both neural and vascular culture models and succeeded in constructing 3D neurovascular tissues with an optimized seeding condition of NSCs, BMECs and MSCs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Reconstituting neurovascular unit based on the close relations between neural stem cells and endothelial cells: an effective method to explore neurogenesis and angiogenesis.Rev Neurosci. 2020 Jan 28;31(2):143-159. doi: 10.1515/revneuro-2019-0023. Rev Neurosci. 2020. PMID: 31539363 Review.
-
Reconstituting neurovascular unit with primary neural stem cells and brain microvascular endothelial cells in three-dimensional matrix.Brain Pathol. 2021 Sep;31(5):e12940. doi: 10.1111/bpa.12940. Epub 2021 Feb 12. Brain Pathol. 2021. PMID: 33576166 Free PMC article.
-
Bioengineering tissue morphogenesis and function in human neural organoids.Semin Cell Dev Biol. 2021 Mar;111:52-59. doi: 10.1016/j.semcdb.2020.05.025. Epub 2020 Jun 12. Semin Cell Dev Biol. 2021. PMID: 32540123 Free PMC article. Review.
-
Reconstituting vascular microenvironment of neural stem cell niche in three-dimensional extracellular matrix.Adv Healthc Mater. 2014 Sep;3(9):1457-64. doi: 10.1002/adhm.201300569. Epub 2014 Feb 12. Adv Healthc Mater. 2014. PMID: 24523050
-
In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells.PLoS One. 2014 Sep 4;9(9):e106346. doi: 10.1371/journal.pone.0106346. eCollection 2014. PLoS One. 2014. PMID: 25187991 Free PMC article.
Cited by
-
Viable human brain microvessels for the study of aging and neurodegenerative diseases.Microvasc Res. 2022 Mar;140:104282. doi: 10.1016/j.mvr.2021.104282. Epub 2021 Nov 20. Microvasc Res. 2022. PMID: 34813858 Free PMC article.
-
Cell Therapies under Clinical Trials and Polarized Cell Therapies in Pre-Clinical Studies to Treat Ischemic Stroke and Neurological Diseases: A Literature Review.Int J Mol Sci. 2020 Aug 27;21(17):6194. doi: 10.3390/ijms21176194. Int J Mol Sci. 2020. PMID: 32867222 Free PMC article. Review.
-
Neural Lineage Differentiation From Pluripotent Stem Cells to Mimic Human Brain Tissues.Front Bioeng Biotechnol. 2019 Dec 6;7:400. doi: 10.3389/fbioe.2019.00400. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31867324 Free PMC article. Review.
-
Cholic Acid Protects In Vitro Neurovascular Units against Oxygen and Glucose Deprivation-Induced Injury through the BDNF-TrkB Signaling Pathway.Oxid Med Cell Longev. 2020 Oct 10;2020:1201624. doi: 10.1155/2020/1201624. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33101581 Free PMC article.
-
Vascularizing the brain in vitro.iScience. 2022 Mar 17;25(4):104110. doi: 10.1016/j.isci.2022.104110. eCollection 2022 Apr 15. iScience. 2022. PMID: 35378862 Free PMC article. Review.
References
-
- Wilhelm, I. & Krizbai, I. A. In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Molecular Pharmaceutics11 (2014). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

