Three-dimensional (3D) culture of adult murine colon as an in vitro model of cryptosporidiosis: Proof of concept
- PMID: 29230047
- PMCID: PMC5725449
- DOI: 10.1038/s41598-017-17304-2
Three-dimensional (3D) culture of adult murine colon as an in vitro model of cryptosporidiosis: Proof of concept
Abstract
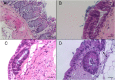
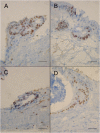
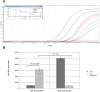
Cryptosporidium parvum is a major cause of diarrheal illness and was recently potentially associated with digestive carcinogenesis. Despite its impact on human health, Cryptosporidium pathogenesis remains poorly known, mainly due to the lack of a long-term culture method for this parasite. Thus, the aim of the present study was to develop a three-dimensional (3D) culture model from adult murine colon allowing biological investigations of the host-parasite interactions in an in vivo-like environment and, in particular, the development of parasite-induced neoplasia. Colonic explants were cultured and preserved ex vivo for 35 days and co-culturing was performed with C. parvum. Strikingly, the resulting system allowed the reproduction of neoplastic lesions in vitro at 27 days post-infection (PI), providing new evidence of the role of the parasite in the induction of carcinogenesis. This promising model could facilitate the study of host-pathogen interactions and the investigation of the process involved in Cryptosporidium-induced cell transformation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

