Synaptotagmin-1 drives synchronous Ca2+-triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment
- PMID: 29230057
- PMCID: PMC5742540
- DOI: 10.1038/s41593-017-0037-5
Synaptotagmin-1 drives synchronous Ca2+-triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment
Abstract
The synaptic vesicle (SV) protein synaptotagmin-1 (Syt1) is the Ca2+ sensor for fast synchronous release. Biochemical and structural data suggest that Syt1 interacts with phospholipids and SNARE complex, but the manner in which these interactions translate into SV fusion remains poorly understood. Using flash-and-freeze electron microscopy, which triggers action potentials with light and coordinately arrests synaptic structures with rapid freezing, we found that synchronous-release-impairing mutations in the Syt1 C2B domain (K325, 327; R398, 399) also disrupt SV-active-zone plasma-membrane attachment. Single action potential induction rescued membrane attachment in these mutants within less than 10 ms through activation of the Syt1 Ca2+-binding site. The rapid SV membrane translocation temporarily correlates with resynchronization of release and paired pulse facilitation. On the basis of these findings, we redefine the role of Syt1 as part of the Ca2+-dependent vesicle translocation machinery and propose that Syt1 enables fast neurotransmitter release by means of its dynamic membrane attachment activities.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

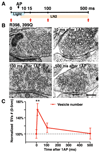

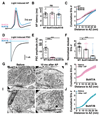
References
-
- Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

