The Toll-Like Receptor 2/6 Agonist, FSL-1 Lipopeptide, Therapeutically Mitigates Acute Radiation Syndrome
- PMID: 29230065
- PMCID: PMC5725477
- DOI: 10.1038/s41598-017-17729-9
The Toll-Like Receptor 2/6 Agonist, FSL-1 Lipopeptide, Therapeutically Mitigates Acute Radiation Syndrome
Abstract
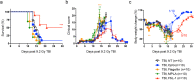
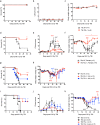
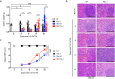
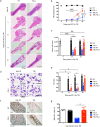
Risks of radiation exposure from nuclear incidents and cancer radiotherapy are undeniable realities. These dangers urgently compel the development of agents for ameliorating radiation-induced injuries. Biologic pathways mediated by myeloid differentiation primary response gene 88 (MyD88), the common adaptor for toll-like receptor (TLR) and Interleukin-1 receptor signaling, are critical for radioprotection. Treating with agonists prior to radiation enhances survival by activating TLR signaling, whereas radiomitigating TLR-activating therapeutics given after exposure are less defined. We examine the radiomitigation capability of TLR agonists and identify one that is superior for its efficacy and reduced toxic consequences compared to other tested agonists. We demonstrate that the synthetic TLR2/6 ligand Fibroblast-stimulating lipopeptide (FSL-1) substantially prolongs survival in both male and female mice when administered 24 hours after radiation and shows MyD88-dependent function. FSL-1 treatment results in accelerated hematopoiesis in bone marrow, spleen and periphery, and augments systemic levels of hematopoiesis-stimulating factors. The ability of FSL-1 to stimulate hematopoiesis is critical, as hematopoietic dysfunction results from a range of ionizing radiation doses. The efficacy of a single FSL-1 dose for alleviating radiation injury while protecting against adverse effects reveals a viable radiation countermeasures agent.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- US Department of Homeland Security. https://www.dhs.gov/topic/nuclear-security (2017).
-
- NIAID-National Institutes of Health. https://www.niaid.nih.gov/topics/radnuc/program/Pages/FocusedResearchDev... (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

