Azadirachtin induced apoptosis in the prothoracic gland in Bombyx mori and a pronounced Ca2+ release effect in Sf9 cells
- PMID: 29230101
- PMCID: PMC5723919
- DOI: 10.7150/ijbs.22175
Azadirachtin induced apoptosis in the prothoracic gland in Bombyx mori and a pronounced Ca2+ release effect in Sf9 cells
Abstract
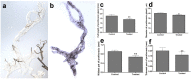
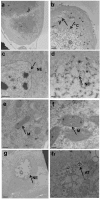
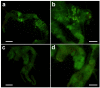
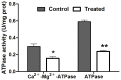
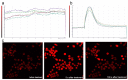
Azadirachtin is a bio-rational insecticide used as an antifeedant and growth disruption agent against many insect species. However, recent studies have shown that there is a potential risk of this compound harming some beneficial insects. In such cases its application does not normally lead to death, but it may result in altered developmental regulation. Therefore, it is essential to obtain toxicological data to understand the mechanism of such sub-lethal effects, especially where they relate to important beneficial insects. Here, we found that azadirachtin could regulate growth and cocooning in silkworms, which may be associated with induced apoptotic effect on the prothoracic gland. However, azadirachtin treatment could not induce apoptosis in the prothoracic gland in vitro, in contrast to the effect of 20-hydroxyecdysone in vitro, which suggesting that the apoptosis might not be direct effect of azadirachtin. Then we examined the activity of Ca2+-Mg2+-ATPase and found that azadirachtin could trigger a significant increase in intracellular Ca2+ release in the Sf9 cell line, which suggested that the calcium signaling pathway might be involved in the process of apoptosis in prothoracic gland and growth regulation in vivo silkworms. Although more evidence is needed to fully understand the mechanism of azadirachtin in perturbing the growth of silkworms, this study provides some toxicological information and highlights the potential risks of azadirachtin in relation to silkworms.
Keywords: Ca2+ release; apoptosis; azadirachtin; growth regulation; prothoracic gland; silkworm.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Abdelgaleil SA, Abbassy MA, Belal A-SH, Rasoul MAA. Bioactivity of two major constituents isolated from the essential oil of Artemisia judaica L. Bioresource technology. 2008;99:5947–50. - PubMed
-
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. - PubMed
-
- Barbosa WF, De Meyer L, Guedes RNC, Smagghe G. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae) Ecotoxicology. 2015;24:130–42. - PubMed
-
- Mosesso P, Bohm L, Pepe G, Fiore M, Carpinelli A, Gäde G. et al. Cytogenetic analyses of Azadirachtin reveal absence of genotoxicity but marked antiproliferative effects in human lymphocytes and CHO cells in vitro. Toxicology letters. 2012;213:361–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

