Cancer nanomedicine: a review of recent success in drug delivery
- PMID: 29230567
- PMCID: PMC5725398
- DOI: 10.1186/s40169-017-0175-0
Cancer nanomedicine: a review of recent success in drug delivery
Abstract
Cancer continues to be one of the most difficult global healthcare problems. Although there is a large library of drugs that can be used in cancer treatment, the problem is selectively killing all the cancer cells while reducing collateral toxicity to healthy cells. There are several biological barriers to effective drug delivery in cancer such as renal, hepatic, or immune clearance. Nanoparticles loaded with drugs can be designed to overcome these biological barriers to improve efficacy while reducing morbidity. Nanomedicine has ushered in a new era for drug delivery by improving the therapeutic indices of the active pharmaceutical ingredients engineered within nanoparticles. First generation nanomedicines have received widespread clinical approval over the past two decades, from Doxil® (liposomal doxorubicin) in 1995 to Onivyde® (liposomal irinotecan) in 2015. This review highlights the biological barriers to effective drug delivery in cancer, emphasizing the need for nanoparticles for improving therapeutic outcomes. A summary of different nanoparticles used for drug delivery applications in cancer are presented. The review summarizes recent successes in cancer nanomedicine in the clinic. The clinical trials of Onivyde leading to its approval in 2015 by the Food and Drug Adminstration are highlighted as a case study in the recent clinical success of nanomedicine against cancer. Next generation nanomedicines need to be better targeted to specifically destroy cancerous tissue, but face several obstacles in their clinical development, including identification of appropriate biomarkers to target, scale-up of synthesis, and reproducible characterization. These hurdles need to be overcome through multidisciplinary collaborations across academia, pharmaceutical industry, and regulatory agencies in order to achieve the goal of eradicating cancer. This review discusses the current use of clinically approved nanomedicines, the investigation of nanomedicines in clinical trials, and the challenges that may hinder development of the nanomedicines for cancer treatment.
Keywords: Clinical trials; Combination treatment; MM-398; Nanoparticles; Oncology; Theranostics; Therapeutics.
Figures



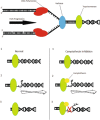

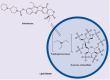


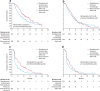
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
