Beta-Blocker Use in Pregnancy and Risk of Specific Congenital Anomalies: A European Case-Malformed Control Study
- PMID: 29230691
- PMCID: PMC5878198
- DOI: 10.1007/s40264-017-0627-x
Beta-Blocker Use in Pregnancy and Risk of Specific Congenital Anomalies: A European Case-Malformed Control Study
Abstract
Introduction: The prevalence of chronic hypertension is increasing in pregnant women. Beta-blockers are among the most prevalent anti-hypertensive agents used in early pregnancy.
Objective: The objective of this study was to investigate whether first-trimester use of beta-blockers increases the risk of specific congenital anomalies in offspring.
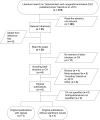
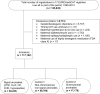
Methods: A population-based case-malformed control study was conducted in 117,122 registrations of congenital anomalies from 17 European Concerted Action on Congenital Anomalies and Twins (EUROCAT) registries participating in EUROmediCAT with data for all or part of the period between 1995 and 2013. Associations previously reported in the literature (signals) were tested and an exploratory analysis was performed to identify new signals. Odds ratios of exposure to any beta-blocker or to a beta-blocker subgroup were calculated for each signal anomaly compared with two control groups (non-chromosomal, non-signal anomalies and chromosomal anomalies). The exploratory analyses were performed for each non-signal anomaly compared with all the other non-signal anomalies.
Results: The signals from the literature (congenital heart defects, oral clefts, neural tube defects and hypospadias) were not confirmed. Our exploratory analysis revealed that multi-cystic renal dysplasia had significantly increased odds of occurring after maternal exposure to combined alpha- and beta-blockers (adjusted odds ratio 3.8; 95% confidence interval 1.3-11.0).
Conclusion: Beta-blocker use in the first trimester of pregnancy was not found to be associated with a higher risk of specific congenital anomalies in the offspring, but a new signal between alpha- and beta-blockers and multi-cystic renal dysplasia was found. Future large epidemiological studies are needed to confirm or refute our findings.
Conflict of interest statement
Funding
This study is a part of the EUROmediCAT project, which was supported by the European Union under the Seventh Framework Programme (HEALTH-F5-2011-260598). EUROCAT registries are funded as described by Greenlees et al. [19]. EUROCAT Northern Netherlands is funded by the Dutch Ministry of Welfare, Health and Sports.
Conflict of interest
Jorieke E.H. Bergman, L. Renée Lutke, Rijk O.B. Gans, Marie-Claude Addor, Ingeborg Barisic, Clara Cavero-Carbonell, Ester Garne, Miriam Gatt, Kari Klungsoyr, Nathalie Lelong, Catherine Lynch, Olatz Mokoroa, Vera Nelen, Amanda J. Neville, Anna Pierini, Hanitra Randrianaivo, Anke Rissmann, Awi Wiesel, Helen Dolk, Maria Loane and Marian K. Bakker have no conflicts of interest directly relevant to the content of this article. David Tucker declares that he is a shareholder in GlaxoSmithKline.
Ethics approval
This study was performed on anonymised patient data and ethics committee approval was therefore not required.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

