Botulinum toxin type A therapy for cervical dystonia
- PMID: 29230798
- PMCID: PMC6486222
- DOI: 10.1002/14651858.CD003633.pub3
Botulinum toxin type A therapy for cervical dystonia
Update in
-
Botulinum toxin type A therapy for cervical dystonia.Cochrane Database Syst Rev. 2020 Nov 12;11(11):CD003633. doi: 10.1002/14651858.CD003633.pub4. Cochrane Database Syst Rev. 2020. PMID: 33180963 Free PMC article.
Abstract
Background: This is an update of a Cochrane Review first published in 2005. Cervical dystonia is the most common form of focal dystonia and is a highly disabling movement disorder characterised by involuntary, usually painful, head posturing. Currently, botulinum toxin type A (BtA) is considered the first line therapy for this condition.
Objectives: To compare the efficacy, safety, and tolerability of botulinum toxin type A (BtA) versus placebo in people with cervical dystonia.
Search methods: To identify studies for this review we searched Cochrane Movement Disorders' Trials Register, CENTRAL, MEDLINE, Embase, reference lists of articles and conference proceedings. All elements of the search, with no language restrictions, were run in October 2016.
Selection criteria: Double-blind, parallel, randomised, placebo-controlled trials (RCTs) of BtA versus placebo in adults with cervical dystonia.
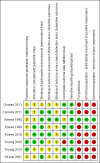
Data collection and analysis: Two review authors independently assessed records, selected included studies, extracted data using a paper pro forma, and evaluated the risk of bias. We resolved disagreements by consensus or by consulting a third review author. We performed meta-analyses using a random-effects model for the comparison of BtA versus placebo to estimate pooled effects and corresponding 95% confidence intervals (95% CI). In addition, we performed preplanned subgroup analyses according to BtA dose used, the BtA formulation used, and the use or not of guidance for BtA injection. The primary efficacy outcome was improvement in cervical dystonia-specific impairment. The primary safety outcome was the proportion of participants with any adverse event.
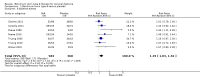
Main results: We included eight RCTs of moderate overall risk of bias, including 1010 participants with cervical dystonia. Six studies excluded participants with poorer responses to BtA treatment, therefore including an enriched population with a higher probability of benefiting from this therapy. Only one trial was independently funded. All RCTs evaluated the effect of a single BtA treatment session, using doses from 150 U to 236 U of onabotulinumtoxinA (Botox), 120 U to 240 U of incobotulinumtoxinA (Xeomin), and 250 U to 1000 U of abobotulinumtoxinA (Dysport).BtA was associated with a moderate-to-large improvement in the participant's baseline clinical status as assessed by investigators, with reduction of 8.06 points in the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS total score) at week 4 after injection (95% CI 6.08 to 10.05; I2 = 0%) compared to placebo, corresponding on average to a 18.7% improvement from baseline. The mean difference (MD) in TWSTRS pain subscore at week 4 was 2.11 (95% CI 1.38 to 2.83; I2 = 0%). Overall, both participants and clinicians reported an improvement of subjective clinical status. There were no differences between groups regarding withdrawals due to adverse events. However, BtA treatment was associated with an increased risk of experiencing an adverse event (risk ratio (RR) 1.19; 95% CI 1.03 to 1.36; I2 = 16%). Dysphagia (9%) and diffuse weakness/tiredness (10%) were the most common treatment-related adverse events (dysphagia: RR 3.04; 95% CI 1.68 to 5.50; I2 = 0%; diffuse weakness/tiredness: RR 1.78; 95% CI 1.08 to 2.94; I2 = 0%). Treatment with BtA was associated with a decreased risk of participants withdrawing from trials. We have moderate certainty in the evidence across all of the aforementioned outcomes.We found no evidence supporting the existence of a clear dose-response relationship with BtA, nor a difference between BtA formulations, nor a difference with use of EMG-guided injection.Due to clinical heterogeneity, we did not pool data regarding health-related quality of life, duration of clinical effect, or the development of secondary non-responsiveness.
Authors' conclusions: We have moderate certainty in the evidence that a single BtA treatment session is associated with a significant and clinically relevant reduction of cervical dystonia-specific impairment, including severity, disability, and pain, and that it is well tolerated, when compared with placebo. There is also moderate certainty in the evidence that people treated with BtA are at an increased risk of developing adverse events, most notably dysphagia and diffuse weakness. There are no data from RCTs evaluating the effectiveness and safety of repeated BtA injection cycles. There is no evidence from RCTs to allow us to draw definitive conclusions on the optimal treatment intervals and doses, usefulness of guidance techniques for injection, the impact on quality of life, or the duration of treatment effect.
Conflict of interest statement
JC, JJF, and CS were investigators in clinical trials in botulinum toxin A and B use in dystonia sponsored by Elan (manufacturer of botulinum toxin type B), Allergan (manufacturer of botulinum toxin type A), and Ipsen (manufacturer of botulinum toxin type A). Searching for studies, selection of studies, data extraction and analysis (including risk of bias), and GRADE assessment were performed by authors (FBR, GSD, MC, REM) who were not trialists. JJF and CS were speakers in symposiums promoted by Elan, Allergan, and Ipsen. APM has received royalties from Ipsen for the use 'LIVEchart' scoring system for botulinum toxin treatment efficacy. He has additionally received consulting fees from Ipsen, Merz (manufacturer of botulinum toxin type A), Eisai (manufacturer of botulinum toxin type B), and Allergan. The same companies have provided for support for travel to meetings for studies or other purposes.
Figures

































Update of
-
Botulinum toxin type A therapy for cervical dystonia.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD003633. doi: 10.1002/14651858.CD003633.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2017 Dec 12;12:CD003633. doi: 10.1002/14651858.CD003633.pub3. PMID: 15674910 Updated.
References
References to studies included in this review
Charles 2012 {published data only}
-
- Brashear A, Truong D, Charles D, Comella C, Hauser RA, Tsui J. A randomized double‐blind, placebo‐controlled study of intramuscular Botox for the treatment of cervical dystonia (CD). Movement Disorders 1998;13 Suppl 2:276 (P4.243).
-
- Charles D, Brashear A, Hauser RA, Li HI, Boo LM, Brin MF, CD 140 Study Group. Efficacy, tolerability, and immunogenicity of onabotulinumtoxinA in a randomized, double‐blind, placebo‐controlled trial for cervical dystonia. Clinical Neuropharmacology 2012;35(5):208‐14. - PubMed
-
- Comella C. Botox for cervical dystonia. Toxins'99 (www.wemove.org). 1999:8.
-
- Hauser RA, Comella C, Brashear A, Truong D, Charles PD, Tsui J. A randomized, multicenter, placebo‐controlled study of original otox (botulinum toxin type A) purified neurotoxin complex for the treatment of cervical dystonia. Movement Disorders 2000;15 Suppl 2:30.
Comella 2011 {published data only}
-
- Comella CL, Jankovic J, Truong DD, Hanschmann A, Grafeon S, on behalf of the U.S. Xeomin Cervical Dystonia Study Group. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN®, botulinumneurotoxin type A, without accessory proteins) in patients with cervical dystonia. Journal of the Neurological Sciences 2011;308(1‐2):103‐9. [DOI: 10.1016/j.jns.2011.05.041.] - DOI - PubMed
-
- Coulonn J‐M. Incobotulinumtoxin A (Xeomin) injected with flexible intervals is a well‐tolerated long‐term treatment of cervical dystonia. Annals of Physical and Rehabilitation Medicine 2013;56:e398.
-
- Evidente VG, Brashear A, Comella CL, Fernandez H, Grafe S, LeDoux MS, et al. IncobotulinumtoxinA (Xeomin; botulinumneurotoxin type A, free from accessory proteins): flexibility of dosing and injection intervals in cervical dystonia. PM&R 2011;3(10S1):S215 (P131).
-
- Evidente VG, Truong D, Jankovic J, Comella CL, Grafe S, Hanschmann A. IncobotulinumtoxinA (Xeomin®) injected for blepharospasm or cervical dystonia according to patient needs is well tolerated. Journal of the Neurological Sciences 2014;346(1‐2):116‐20. [DOI: 10.1016/j.jns.2014.08.004; MEDLINE: ] - DOI - PubMed
-
- Fernandez H, Truong D, Asmus F, Hanschmann A, LeDoux M. IncobotulinumtoxinA (Xeomin®) remains well tolerated across flexible inter‐injection intervals of 6‐20 weeks in the treatment of cervical dystonia. Neurology 2012;78(Meeting Abstracts 1):P01.219.
Greene 1990 {published data only}
-
- Greene P, Kang U, Fahn S, Brin M, Moskowitz C, Flaster E. Double‐blind, placebo‐controlled study of botulinum toxin injection for torticollis. Neurology 1988;38 Suppl 1:244. - PubMed
-
- Greene P, Kang U, Fahn S, Brin M, Moskowitz C, Flaster E. Double‐blind, placebo‐controlled trial of botulinum toxin injections for the treatment of spasmodic torticollis. Neurology 1990;40(8):1213‐8. - PubMed
Poewe 1998 {published data only}
-
- Poewe W. Dysport for Cervical Dystonia. Toxins'99 (www.wemove.org). 1999:10.
-
- Poewe W, Brans J, Kessler K, Kanovsky P, Odergren T, Aramideh M, et al. Dysport for cervical dystonia. Movement Disorders 2000;15 Suppl 2:7.
-
- Poewe W, Deuschl G, Nebe A, Feifel E, Wissel J, Benecke R, et al. What is the optimal dose of botulinum toxin A in the treatment of cervical dystonia? Results of a double blind, placebo controlled, dose ranging study using Dysport. German Dystonia Study Group. Journal of Neurology, Neurosurgery and Psychiatry 1998;64(1):13‐7. - PMC - PubMed
Poewe 2016 {published data only}
-
- Poewe W, Burbaud P, Castelnovo G, Jost WH, Ceballos‐Baumann AO, Banach M, et al. Efficacy and safety of abobotulinumtoxinA liquid formulation in cervical dystonia: a randomized‐controlled trial. Movement Disorders 2016 [Epub ahead of print]; Vol. 21. - PubMed
Truong 2005 {published data only}
-
- Coleman CM, Chang SF, Copley‐Merriman C, Hubble J, Masaquel C. Health‐related quality of life improvements with dysport in cervical dystonia. PM&R 2011;3 Suppl.1(10):S190 (P41).
-
- Hauser RA, Truong D, Hubble J, Coleman C, Beffy JL, Chang S, Picaut P. AbobotulinumtoxinA (Dysport) dosing in cervical dystonia: an exploratory analysis of two large open‐label extension studies. Journal of Neural Transmission 2013;120(2):299‐307. [DOI: 10.1007/s00702-012-0872-1; PUBMED: 22878514] - DOI - PubMed
-
- Jen M, Kurth H, Iheanacho I, Dinet J, Gabriel S, Wasiak R, et al. Improvement of SF‐36 scores in cervical dystonia patients ‐ is there a treatment effect when evaluating subscales?. Basal Ganglia 2014;126:1‐6.
-
- Truong D, Duane DD, Jankovic J, Singer C, Seeberger LC, Comella CL, et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: results of the first US randomized, double‐blind, placebo‐controlled study. Movement Disorders 2005;20(7):783‐91. - PubMed
-
- Truong DD, Duane DD, Jankovic J, Singer C, Comella CL. The efficacy and safety of Dysport (botulinumtype A toxin) in cervical dystonia: results of the first US study. Movement Disorders 2002;17 Suppl 5:S306 (P1007). - PubMed
Truong 2010 {published data only}
-
- Mordin M, Masaquel C, Abbott C, Copley‐Merriman C. Factors affecting the health‐related quality of life of patients with cervical dystonia and impact of treatment with abobotulinumtoxinA (Dysport): results from a randomised, double‐blind, placebo‐controlled study . BMJ Open 2014;4(10):e005150. [DOI: 10.1136/bmjopen-2014-005150; PUBMED: 25324317] - DOI - PMC - PubMed
-
- Truong D, Brodsky M, Lew M, Brashear A, Jankovic J, Molho E, et al. Global Dysport Cervical Dystonia Study Group. Long‐term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Parkinsonism & Related Disorders 2010;16(5):316‐23. [DOI: 10.1016/j.parkreldis.2010.03.002; MEDLINE: ] - DOI - PubMed
Wissel 2001 {published data only}
-
- Kanovsky P, Ruzicka E, Jech R, Roth J, Schnider P, Auff E, et al. Standardized dose of 500 Mu of Dysport for cervical dystonia: results of a prospective, randomized, double‐blind, placebo‐controlled study. Movement Disorders. 2000; Vol. 15 Suppl 2:31.
-
- Poewe W. Dysport for cervical cystonia. Toxins'99 (www.wemove.org). 1999:10.
-
- Poewe W, Brans J, Kessler K, Kanovsky P, Odergren T, Aramideh M, et al. Dysport for cervical dystonia. Movement Disorders. 2000; Vol. 15 Suppl 2:7.
-
- Wissel J, Kanovsky P, Ruzicka E, Bares M, Hortova H, Streitova H, et al. Efficacy and safety of a standardised 500 unit dose of Dysport (clostridium botulinum toxin type A haemaglutinin complex) in a heterogeneous cervical dystonia population: results of a prospective, multicentre, randomised, double‐blind, placebo‐controlled, parallel group study. Journal of Neurology 2001;248 (12):1073‐8. - PubMed
References to studies excluded from this review
Blackie 1990 {published data only}
-
- Turjanski N, Blackie J, Purves A, Hambleton P, Lees AJ. Botulinus toxin in the treatment of spasmodic torticollis long‐term results. Journal of Neurology. 2nd Congress of European Neurological Society, 1990.
Buchman 1994 {published data only}
-
- Buchman AS, Comella CL, Stebbins GT, Weinstein SL. Determining a dose‐ effect curve for botulinum toxin in the sternocleidomastoid muscle in cervical dystonia. Clinical Neuropharmacology 1994;17(2):188‐95.
Gelb 1989 {published data only}
-
- Gelb DJ, Arbor A, Don M, Yoshimura RKO, Lowenstein DH, Aminoff MJ. Change in pattern of muscle activity following botulinum toxin injections for torticollis. Neurology 1989;39 Suppl 1:320. - PubMed
-
- Gelb DJ, Lowenstein DH, Aminoff MJ. Botulinum toxin in the treatment of spasmodic torticollis. Neurology. 1988; Vol. 38 Suppl 2:244. - PubMed
-
- Gelb DJ, Lowenstein DH, Aminoff MJ. Controlled trial of botulinum toxin injections in the treatment of spasmodic torticollis. Neurology 1989;39 (1):80‐4. - PubMed
Koller 1990 {published data only}
-
- Koller W, Vetere‐Overfield B, Gray C, Dubinsky R. Failure of fixed‐dose, fixed muscle injection of botulinum toxin in torticollis. Clinical Neuropharmacology 1990;13 (4):355‐8. - PubMed
Lange 1991 {published data only}
-
- Lange DJ, Rubin M, Greene PE, Kang J, Moskowitz CB, Brin MF, et al. Distant effects of locally injected botulinum toxin: a double‐blind study of single fiber EMG changes. Muscle and Nerve 1991;14:672‐5. - PubMed
Lorentz 1991 {published data only}
-
- Lorentz IT, Subramaniam SS, Yiannikas C. Treatment of idiopathic spasmodic torticollis with botulinum toxin A: a double‐blind study on twenty‐three patients. Movement Disorders 1991;6 (2):145‐50. - PubMed
Lu 1995 {published data only}
-
- Lu CS, Chen RS, Tsai CH. Double‐blind, placebo‐controlled study of botulinum toxin injections in the treatment of cervical dystonia. Journal of the Formosan Medical Association 1995;94 (4):189‐92. - PubMed
Maurri 1990 {published data only}
-
- Maurri S, Brogelli S. Botulinum toxin therapy for blepharospasm, hemifacial spasm and spasmodic torticollis: clinical studies with a follow‐up of 40 months. Movement Disorders 1990;5 Suppl 1:73 (P262).
Moore 1991 {published data only}
Ostergaard 1994 {published data only}
-
- Ostergaard L, Fuglsang‐Frederiksen A, Werdelin L, Sjo O, Winkel H. Quantitative EMG in botulinum toxin treatment of cervical dystonia. A double‐blind, placebo‐controlled study. Electroencephalography and Clinical Neurophysiology 1994;93(6):434‐9. - PubMed
Perlmutter 1989 {published data only}
-
- Perlmutter JS, Tempel LW, Burde R. Double‐blind, placebo‐controlled, crossover trial of botulinum‐A toxin for torticollis. Neurology 1989;39 Suppl 1:352.
Relja 1993 {published data only}
-
- Relja M, Petravic D. Botulinum toxin type A injections for cervical dystonia: a double‐blind, placebo controlled study. The Canadian Journal of Neurological Sciences 1993;20 Suppl 4:S185 (6‐17‐01).
Tsui 1986 {published data only}
-
- Tsui JK, Eisen A, Stoessl AJ, Calne S, Calne DB. Double‐blind study of botulinum toxin in spasmodic torticollis. Lancet 1996;2 (2):245‐7. - PubMed
Tsui 1988 {published data only}
-
- Tsui J, Eisen A, Calne DB. Botulinum toxin in spasmodic torticollis. Advances in Neurology. Stanley Fahn et al. Vol. 50: Dystonia 2, New York: Raven Press, 1988:593‐7. - PubMed
Yoshimura 1990 {published data only}
-
- Yoshimura D, Aminoff M, Gelb D. Long term follow‐up of patients treated with botulinum toxin for spasmodic torticollis. Movement Disorders 1990;5 (Suppl 1):75 (P268).
Additional references
Abrams 2005
-
- Abrams KR, Gillies CL, Lambert PC. Meta‐analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24:3823‐44. - PubMed
Albanese 2011
-
- Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. European Journal of Neurology 2011;18:5‐18. - PubMed
Albanese 2013
Altman 2002
Antonucci 2008
Balint 2015
-
- Balint B, Bhatia KP. Isolated and combined dystonia syndromes ‐ an update on new genes and their phenotypes. European Journal of Neurology 2015;22(4):610‐7. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Boroff 1975
-
- Boroff DA, Chen GS. On the question of permeability of the blood–brain barrier to botulinum toxin. International Archives of Allergy and Applied Immunology 1975;48:495–504. - PubMed
Brożek 2006
Carpenter 2013
-
- Carpenter J, Kenward M. Multiple Imputation and its Application. Wiley, 2013.
Chan 1991
-
- Chan J, Brin MF, Fanh S. Idiopathic cervical dystonia: clinical characteristics. Movement Disorders 1991;6:119‐26. - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc , 1988.
Comella 2015
Corbett 2014
-
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5(1):79‐85. - PubMed
de Paiva 1999
-
- Paiva A, Meunier FA, Molgó J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proceedings of the National Academy of Sciences of the United States of America 1999;96:3200–5. - PMC - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Defazio 2013
Duarte 2016
Duchen 1971
-
- Duchen LW. An electron microscopic study of the changes induced by botulinum toxin in the motor end‐plates of slow and fast skeletal muscle fibres of the mouse. Journal of the Neurological Sciences 1971;14:47‐60. - PubMed
Edwards 2000
-
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356(9237):1255‐9. - PubMed
ESDE 2000
-
- Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. Journal of Neurology 2000;247:787‐92. - PubMed
Filippi 1993
-
- Filippi GM, Errico P, Santarelli R, Bagolini B, Manni E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Oto‐laryngologica 1993;113:400–4. - PubMed
Follmann 1992
-
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. - PubMed
Foltz 1959
-
- Foltz EL, Knopp LM, Ward AA. Experimental spasmodic torticollis. Journal of Neurosurgery 1959;16:55‐72. - PubMed
Frevert 2010
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version assessed 3 January 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. - PubMed
Hallett 1998
-
- Hallett M. The neurophysiology of dystonia. Archives of Neurology 1998;55(5):601‐3. - PubMed
Hedges 1985
-
- Hedges LV, Olkin I. Statistical Methods for Meta‐Analysis. San Diego, California: Academic Press, Inc, 1985.
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holland 1981
-
- Holland RL, Brown MC. Nerve growth in botulinum toxin poisoned muscles. Neuroscience 1981;6:1167–79. - PubMed
Imberger 2016
Jahnanshani 1990
-
- Jahnanshani M, Marion M‐H, Marsden CD. Natural history of adult‐onset idiopathic torticollis. Archives of Neurology 1990;47:548‐52. - PubMed
Jakobsen 2014
Jankovic 2004
Jankovic 2006
-
- Jankovic J, Tsui J, Bergeron C. Prevalence of cervical dystonia and spasmodic torticollis in the United States general population. Parkinsonism & Related Disorders 2007;13(7):411–6. - PubMed
Juzans 1996
-
- Juzans P, Comella J, Molgo J, Faille L, Angaut‐Petit D. Nerve terminal sprouting in botulinum type‐A treated mouse levator auris longus muscle. Neuromuscular Disorders : NMD 1996;6(3):177‐85. - PubMed
Liberati 2009
Lundh 2017
Macefield 2014
Marques 2016
Matak 2014
-
- Matak I, Lacković Z. Botulinum toxin A, brain and pain. Progress in Neurobiology 2014;119‐120:39–59. - PubMed
Matak 2015
-
- Matak I, Lacković Z. Botulinum neurotoxin type A: actions beyond SNAP‐25?. Toxicology 2015;335:79‐84. - PubMed
O'Brien 1979
-
- O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549‐56. - PubMed
Palomar 2012
-
- Palomar FJ, Mir P. Neurophysiological changes after intramuscular injection of botulinum toxin. Clinical Neurophysiology 2012;123(23):54–60. - PubMed
Pellizzari 1999
Peters 2006
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta‐analysis. JAMA 2006;295(6):676‐80. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Centre, The Cochrane Collaboration, 2014.
Rosales 1996
-
- Rosales RL, Arimura K, Takenaka S, Osame M. Extrafusal and intrafusal muscle effects in experimental botulinum toxin‐A injection. Muscle & Nerve 1996;19:488–96. - PubMed
Rosales 2010
-
- Rosales RL, Dressler D. On muscle spindles, dystonia and botulinum toxin. European Journal of Neurology 2010;17:71–80. - PubMed
Rubin 1991
-
- Rubin DB, Schenker N. Multiple imputation in health‐care databases: an overview and some applications. Statistics in Medicine 1991;10:585–98. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Simpson 2004
-
- Simpson LL. Identification of the major steps in botulinum toxin action. Annual Review of Pharmacology and Toxicology 2004;44:167‐93. - PubMed
Simpson 2016
-
- Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016;10:1818‐26. - PMC - PubMed
Smeeth 1999
Stata 2015 [Computer program]
-
- StataCorp. Stata Statistical Software: release 14. Version accessed 3 January 2017. StataCorp, 2015.
Steeves 2012
-
- Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta‐analysis. Movement Disorders 2012;27(14):1789‐96. - PubMed
Sterne 2001
-
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tarsy 1997
-
- Tarsy D. Comparison of clinical rating scales in treatment of cervical dystonia with botulinum toxin. Movement Disorders 1997;12(1):100‐2. - PubMed
Tarsy 2006
-
- Tarsy D, Simon DK. Dystonia. New England Journal of Medicine 2006;355:818‐29. - PubMed
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa ˙manual.pdf 2011.
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. Trial Sequential Analysis. Version 0.9 Beta. Version accessed 3 January 2017. Copenhagen: Copenhagen Trial Unit, 2011.
Tugwell 2007
Walker 2014
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. - PubMed
Wetterslev 2009
Wiebe 2006
-
- Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. Journal of Clinical Epidemiology 2006;59(4):342‐53. - PubMed
References to other published versions of this review
Costa 2000
-
- Costa J, Ferreira JJ, Sampaio C. Botulinum toxin type A for the treatment of cervical dystonia: a systematic review. Movement Disorders 2000;15 Suppl 3:29.
Costa 2005
Ferreira 1999
-
- Ferreira JJ, Costa J, Sampaio C. Botulinum toxin type A for the treatment of cervical dystonia: a systematic review. European Journal of Neurology 1999;6 Suppl 3:76.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

