Dynamic autoregulation of cerebral blood flow measured non-invasively with fast diffuse correlation spectroscopy
- PMID: 29231781
- PMCID: PMC5951022
- DOI: 10.1177/0271678X17747833
Dynamic autoregulation of cerebral blood flow measured non-invasively with fast diffuse correlation spectroscopy
Abstract
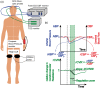
Cerebral autoregulation (CA) maintains cerebral blood flow (CBF) in the presence of systemic blood pressure changes. Brain injury can cause loss of CA and resulting dysregulation of CBF, and the degree of CA impairment is a functional indicator of cerebral tissue health. Here, we demonstrate a new approach to noninvasively estimate cerebral autoregulation in healthy adult volunteers. The approach employs pulsatile CBF measurements obtained using high-speed diffuse correlation spectroscopy (DCS). Rapid thigh-cuff deflation initiates a chain of responses that permits estimation of rates of dynamic autoregulation in the cerebral microvasculature. The regulation rate estimated with DCS in the microvasculature (median: 0.26 s-1, inter quartile range: 0.19 s-1) agrees well (R = 0.81, slope = 0.9) with regulation rates measured by transcranial Doppler ultrasound (TCD) in the proximal vasculature (median: 0.28 s-1, inter quartile range: 0.10 s-1). We also obtained an index of systemic autoregulation in concurrently measured scalp microvasculature. Systemic autoregulation begins later than cerebral autoregulation and exhibited a different rate (0.55 s-1, inter quartile range: 0.72 s-1). Our work demonstrates the potential of diffuse correlation spectroscopy for bedside monitoring of cerebral autoregulation in the microvasculature of patients with brain injury.
Keywords: Cerebral blood flow; cerebral autoregulation; intrinsic optical imaging; neurocritical care; noninvasive monitoring.
Figures
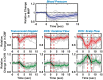

 ,
n = 8). Outliers (not included in the analysis, see
text) are marked with red asterisk (
,
n = 8). Outliers (not included in the analysis, see
text) are marked with red asterisk ( ). Horizontal red
line in each box represents the median of measurements, while horizontal
blue lines represent the 75th and 25th quartile.
). Horizontal red
line in each box represents the median of measurements, while horizontal
blue lines represent the 75th and 25th quartile.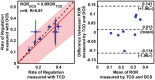
References
-
- Strandgaard S, Paulson OB. Cerebral autoregulation. Stroke 1984; 15: 413–416. - PubMed
-
- Budohoski KP, Czosnyka M, Kirkpatrick PJ, et al. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat Rev Neurol 2013; 9: 152–163. - PubMed
-
- Atkins ER, Brodie FG, Rafelt SE, et al. Dynamic cerebral autoregulation is compromised acutely following mild ischaemic stroke but not transient ischaemic attack. Cerebrovasc Dis 2009; 29: 228–235. - PubMed
-
- Dawson SL, Blake MJ, Panerai RB, et al. Dynamic but not static cerebral autoregulation is impaired in acute ischaemic stroke. Cerebrovasc Dis 2000; 10: 126–132. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

