The US Food and Drug Administration's tentative approval process and the global fight against HIV
- PMID: 29232052
- PMCID: PMC5810328
- DOI: 10.1002/jia2.25019
The US Food and Drug Administration's tentative approval process and the global fight against HIV
Abstract
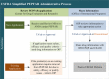
Introduction: In 2004, the US government began to utilize the Food and Drug Administration's (USFDA) tentative approval process (tFDA) as a basis to determine which HIV drugs are appropriate to be purchased and used in resource-constrained settings. This process permits products that are not approved for marketing in the US, including medicines with active patents or marketing restrictions in the US, to be purchased and distributed in resource-constrained settings. Although the tFDA was originally intended to support the United States' President's Emergency Plan for AIDS Relief (PEPFAR), the USFDA list has become a cornerstone of international HIV programmes that support procurement of ARVs, such as the World Health Organization and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. Our objective in this article is to help the global HIV policy makers and implementers of HIV programmes better understand the benefits and limitations of the tFDA by providing an in-depth review of the relevant legal and regulatory processes.
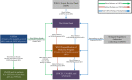
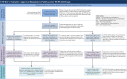
Discussion: USFDA's dedicated tFDA process for ARVs used by the PEPFAR programme has a wide impact globally; however, the implementation and the regulatory processes governing the programme have not been thoroughly described in the medical literature. This paper seeks to help stakeholders better understand the legal and regulatory aspects associated with review of ARVs under the tFDA by describing the following: (1) the tFDA and its importance to global ARV procurement; (2) the regulatory pathways for applications under tFDA for the PEPFAR programme, including modifications to applications, review timelines and costs; (3) the role of US patents, US marketing exclusivity rights, and the Medicines Patents Pool in tFDA; and (4) an overview of how applications for PEPFAR programme are processed through the USFDA. We also provide a case study of a new ARV, tenofovir alafenamide fumarate (TAF), not yet reviewed by USFDA for PEPFAR use.
Conclusions: In this paper, we describe the importance and implementation of USFDA's tentative approval process to review ARVs for resource-constrained settings. We also highlight the impact of patents and exclusivities on review of HIV drugs under tFDA and illustrate the concepts using a new HIV drug as an example.
Keywords: AIDS; FDA; HIV; PEPFAR; TAF; USFDA; Global Health; tentative approval.
© 2017 The Authors. Journal of the International AIDS Society published by John Wiley & sons Ltd on behalf of the International AIDS Society.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) . Fact Sheet 2016. 2016. [Accessed: 10 November 2016]; Available from: http://www.unaids.org/en/resources/fact-sheet.
-
- Padian NS, Holmes CB, McCoy SI, Lyerla R, Bouey PD, Goosby EP. Implementation science for the US President's Emergency Plan for AIDS Relief (PEPFAR). J Acquir Immune Defic Syndr. 2011;56(3):199–203. - PubMed
-
- United States Congress . Tom Lantos and Henry J. Hyde United States Global Leadership Against HIV/AIDS, Tuberculosis, and Malaria Reauthorization Act of 2008, in 22 U.S.C. 7601. 2008: United States. [Accessed: 14 June 2016]; Available from: http://www.pepfar.gov/documents/organization/108294.pdf.
-
- Venkatesh KK, Mayer KH, Carpenter CC. Low‐cost generic drugs under the President's Emergency Plan for AIDS Relief drove down treatment cost; more are needed. Health Aff (Millwood). 2012;31(7):1429–38. - PubMed
-
- US Food and Drug Administration . Drugs@FDA Glossary of Terms – Tentative Approval. 2012. [Accessed: 1 September 2015]; Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ucm079436.htm#tentative.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

