Catalytic Asymmetric Synthesis of Trifluoromethylated γ-Amino Acids through the Umpolung Addition of Trifluoromethyl Imines to Carboxylic Acid Derivatives
- PMID: 29232488
- PMCID: PMC6408948
- DOI: 10.1002/anie.201710915
Catalytic Asymmetric Synthesis of Trifluoromethylated γ-Amino Acids through the Umpolung Addition of Trifluoromethyl Imines to Carboxylic Acid Derivatives
Abstract
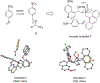
Novel cinchona alkaloid derived chiral phase-transfer catalysts enabled the highly chemo-, regio-, diastereo-, and enantioselective umpolung addition of trifluoromethyl imines to α,β-unsaturated N-acyl pyrroles. With a catalyst loading ranging from 0.2 to 5.0 mol %, this new catalytic asymmetric transformation provides facile and high-yielding access to highly enantiomerically enriched chiral trifluoromethylated γ-amino acids and γ-lactams.
Keywords: C−C bond formation; amino acids; asymmetric catalysis; phase-transfer catalysis; umpolung.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
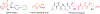

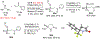
Similar articles
-
Catalytic Asymmetric Synthesis of Chiral γ-Amino Ketones via Umpolung Reactions of Imines.J Am Chem Soc. 2016 Dec 14;138(49):15817-15820. doi: 10.1021/jacs.6b09754. Epub 2016 Nov 30. J Am Chem Soc. 2016. PMID: 27960300 Free PMC article.
-
Direct Catalytic Asymmetric Synthesis of Trifluoromethylated γ-Amino Esters/Lactones via Umpolung Strategy.J Org Chem. 2019 Jan 18;84(2):994-1005. doi: 10.1021/acs.joc.8b02893. Epub 2019 Jan 8. J Org Chem. 2019. PMID: 30543752 Free PMC article.
-
Synthesis of α-amino acid derivatives and peptides via enantioselective addition of masked acyl cyanides to imines.J Am Chem Soc. 2014 Nov 19;136(46):16148-51. doi: 10.1021/ja510135t. Epub 2014 Nov 7. J Am Chem Soc. 2014. PMID: 25366558 Free PMC article.
-
The direct catalytic asymmetric mannich reaction.Acc Chem Res. 2004 Feb;37(2):102-12. doi: 10.1021/ar030231l. Acc Chem Res. 2004. PMID: 14967057 Review.
-
Catalytic asymmetric umpolung reactions of imines via 2-azaallyl anion intermediates.Org Biomol Chem. 2021 May 19;19(19):4193-4212. doi: 10.1039/d1ob00409c. Org Biomol Chem. 2021. PMID: 33870977 Review.
Cited by
-
2-Azadienes as Enamine Umpolung Synthons for the Preparation of Chiral Amines.Synlett. 2019 Jul;30(11):1253-1268. doi: 10.1055/s-0037-1611770. Epub 2019 Mar 26. Synlett. 2019. PMID: 33731976 Free PMC article.
-
Pd-Catalyzed Allylation of Imines to Access α-CF3-Substituted α-Amino Acid Derivatives.European J Org Chem. 2019 Nov 14;2019(42):7122-7127. doi: 10.1002/ejoc.201901272. Epub 2019 Oct 9. European J Org Chem. 2019. PMID: 31798337 Free PMC article.
-
Enantioselective Synthesis of α-Trifluoromethyl Amines via Biocatalytic N-H Bond Insertion with Acceptor-Acceptor Carbene Donors.J Am Chem Soc. 2022 Feb 16;144(6):2590-2602. doi: 10.1021/jacs.1c10750. Epub 2022 Feb 2. J Am Chem Soc. 2022. PMID: 35107997 Free PMC article.
-
Asymmetric Catalytic Ketimine Mannich Reactions and Related Transformations.Catalysts. 2021 Jun;11(6):712. doi: 10.3390/catal11060712. Epub 2021 Jun 7. Catalysts. 2021. PMID: 34745653 Free PMC article.
-
Synthesis of α-CF3-proline derivatives by means of a formal (3 + 2)-cyclisation between trifluoropyruvate imines and Michael acceptors.Org Biomol Chem. 2019 Jun 12;17(23):5731-5735. doi: 10.1039/c9ob01134j. Org Biomol Chem. 2019. PMID: 31149695 Free PMC article.
References
-
-
For selected examples of biologically interesting natural products and analogues, see:
Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH, J. Nat. Prod 2001, 64, 907–910;
Maderna A, Doroski M, Subramanyam C, Porte A, Leverett CA, Vetelino BC, Chen Z, Risley H, Parris K, Pandit J, Varghese AH, Shanker S, Song C, Sukuru SCK, Farley KA, Wagenaar MM, Shapiro MJ, Musto S, Lam M-H, Loganzo F, O’Donnell CJ, J. Med. Chem 2014, 57, 10527–10543;
Colombo R, Wang Z, Han J, Balachandran R, Daghestani HN, Camarco DP, Vogt A, Day BW, Mendel D, Wipf P, J. Org. Chem 2016, 81, 10302–10320;
Khalil MW, Sasse F, Lünsdorf H, Elnakady YA, Reichenbach H, Chembiochem 2006, 7, 678–683;
Palomo C, Oiarbide M, García JM, González A, Pazos R, Odriozola JM, Bañuelos P, Tello M, Linden A, J. Org. Chem 2004, 69, 4126–4134.
-
-
-
For selected examples of medical agents, see:
Gulder TAM, Moore BS, Angew. Chem. Int. Ed 2010, 49, 9346–9367; Angew. Chem. 2010, 122, 9534–9556;
Froestl W, Adv. Pharmacol 2010, 58, 19–62;
Silverman RB, Angew. Chem. Int. Ed 2008, 47, 3500–3504; Angew. Chem. 2008, 120, 3552–3556;
Al-Majed A, Profiles of Drug Substances, Excipients and Related Methodology 2010, 35, 309–345.
-
-
-
For selected reviews on the asymmetric synthesis of γ-amino acid/lactam derivatives see:
Ordóñez M, Cativiela C, Tetrahedron: Asymmetry 2007, 18, 3–99;
Ordóñez M, Cativiela C, Romero-Estudillo I, Tetrahedron: Asymmetry 2016, 27, 999–1055;
Caruano J, Muccioli GG, Robiette R, Org. Biomol. Chem 2016, 14, 10134–10156.
-
-
- Pesenti C, Arnone A, Bellosta S, Bravo P, Canavesi M, Corradi E, Frigerio M, Meille SV, Monetti M, Panzeri W, Viani F, Venturini R, Zanda M, Tetrahedron 2001, 57, 6511–6522;
- Chaume G, Van Severen M-C, Ricard L, Brigaud T, J. Fluorine Chem 2008, 129, 1104–1109;
- Berger AA, Völler J-S, Budisa N, Koksch B, Acc. Chem. Res 2017, 50, 2093–2103. - PubMed
-
- Li H, Wang Y, Tang L, Deng L, J. Am. Chem. Soc 2004, 126, 9906–9907; - PubMed
- Li H, Wang Y, Tang L, Wu F, Liu X, Guo C, Foxman BM, Deng L, Angew. Chem. Int. Ed 2005, 44, 105–108; Angew. Chem. 2005, 117, 107–110; - PubMed
- Okino T, Hoashi Y, Furukawa T, Xu X, Takemoto Y, J. Am. Chem. Soc 2005, 127, 119–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

