[Carboxyl-11 C]Labelling of Four High-Affinity cPLA2α Inhibitors and Their Evaluation as Radioligands in Mice by Positron Emission Tomography
- PMID: 29232493
- PMCID: PMC6077846
- DOI: 10.1002/cmdc.201700697
[Carboxyl-11 C]Labelling of Four High-Affinity cPLA2α Inhibitors and Their Evaluation as Radioligands in Mice by Positron Emission Tomography
Abstract
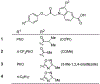
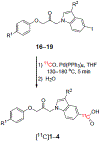
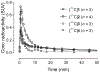
Cytosolic phospholipase A2α (cPLA2α) may play a critical role in neuropsychiatric and neurodegenerative disorders associated with oxidative stress and neuroinflammation. An effective PET radioligand for imaging cPLA2α in living brain might prove useful for biomedical research, especially on neuroinflammation. We selected four high-affinity (IC50 2.1-12 nm) indole-5-carboxylic acid-based inhibitors of cPLA2α, namely 3-isobutyryl-1-(2-oxo-3-(4-phenoxyphenoxy)propyl)-1H-indole-5-carboxylic acid (1); 3-acetyl-1-(2-oxo-3-(4-(4-(trifluoromethyl)phenoxy)phenoxy)propyl)-1H-indole-5-carboxylic acid (2); 3-(3-methyl-1,2,4-oxadiazol-5-yl)-1-(2-oxo-3-(4-phenoxyphenoxy)propyl)-1H-indole-5-carboxylic acid (3); and 3-(3-methyl-1,2,4-oxadiazol-5-yl)-1-(3-(4-octylphenoxy)-2-oxopropyl)-1H-indole-5-carboxylic acid (4), for labelling in carboxyl position with carbon-11 (t1/2 =20.4 min) to provide candidate PET radioligands for imaging brain cPLA2α. Compounds [11 C]1-4 were obtained for intravenous injection in adequate overall yields (1.1-5.5 %) from cyclotron-produced [11 C]carbon dioxide and with moderate molar activities (70-141 GBq μmol-1 ) through the use of Pd0 -mediated [11 C]carbon monoxide insertion on iodo precursors. Measured logD7.4 values were within a narrow moderate range (1.9-2.4). After intravenous injection of [11 C]1-4 in mice, radioactivity uptakes in brain peaked at low values (≤0.8 SUV) and decreased by about 90 % over 15 min. Pretreatments of the mice with high doses of the corresponding non-radioactive ligands did not alter brain time-activity curves. Brain uptakes of radioactivity after administration of [11 C]1 to wild-type and P-gp/BCRP dual knock-out mice were similar (peak 0.4 vs. 0.5 SUV), indicating that [11 C]1 and others in this structural class, are not substrates for efflux transporters.
Keywords: cPLA2α; carbon-11; carbonylation; neuroinflammation; radiopharmaceuticals.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




Similar articles
-
1-Heteroaryl-3-phenoxypropan-2-ones as inhibitors of cytosolic phospholipase A₂α and fatty acid amide hydrolase: Effect of the replacement of the ether oxygen with sulfur and nitrogen moieties on enzyme inhibition and metabolic stability.Bioorg Med Chem. 2015 May 15;23(10):2579-92. doi: 10.1016/j.bmc.2015.03.033. Epub 2015 Mar 21. Bioorg Med Chem. 2015. PMID: 25862211
-
Investigations on the metabolic stability of cytosolic phospholipase A2α inhibitors with 1-indolylpropan-2-one structure.Chem Biol Interact. 2013 Nov 25;206(2):356-63. doi: 10.1016/j.cbi.2013.10.005. Epub 2013 Oct 10. Chem Biol Interact. 2013. PMID: 24120545
-
1-(3-biaryloxy-2-oxopropyl)indole-5-carboxylic acids and related compounds as dual inhibitors of human cytosolic phospholipase A2α and fatty acid amide hydrolase.ChemMedChem. 2011 Mar 7;6(3):544-9. doi: 10.1002/cmdc.201000473. Epub 2011 Jan 21. ChemMedChem. 2011. PMID: 21259444
-
Targeting Cytosolic Phospholipase A2α for Novel Anti-Inflammatory Agents.Curr Med Chem. 2018;25(21):2418-2447. doi: 10.2174/0929867325666180117103919. Curr Med Chem. 2018. PMID: 29345571 Review.
-
(11)C[double bond, length as m-dash]O bonds made easily for positron emission tomography radiopharmaceuticals.Chem Soc Rev. 2016 Aug 22;45(17):4708-26. doi: 10.1039/c6cs00310a. Chem Soc Rev. 2016. PMID: 27276357 Free PMC article. Review.
Cited by
-
Copper(I)-Mediated 11C-Carboxylation of (Hetero)arylstannanes.ACS Omega. 2020 Apr 2;5(14):8242-8250. doi: 10.1021/acsomega.0c00524. eCollection 2020 Apr 14. ACS Omega. 2020. PMID: 32309734 Free PMC article.
-
A General and Scalable Synthesis of Polysubstituted Indoles.Molecules. 2020 Nov 28;25(23):5595. doi: 10.3390/molecules25235595. Molecules. 2020. PMID: 33260745 Free PMC article.
-
[11C]Carbon monoxide: advances in production and application to PET radiotracer development over the past 15 years.EJNMMI Radiopharm Chem. 2019 Sep 18;4(1):25. doi: 10.1186/s41181-019-0073-4. EJNMMI Radiopharm Chem. 2019. PMID: 31659516 Free PMC article. Review.
-
Small-molecule inhibitors as potential therapeutics and as tools to understand the role of phospholipases A2.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):941-956. doi: 10.1016/j.bbalip.2018.08.009. Epub 2018 Aug 23. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30905350 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

