Napsin A levels in epithelial lining fluid as a diagnostic biomarker of primary lung adenocarcinoma
- PMID: 29233112
- PMCID: PMC5727880
- DOI: 10.1186/s12890-017-0534-z
Napsin A levels in epithelial lining fluid as a diagnostic biomarker of primary lung adenocarcinoma
Abstract
Background: It is crucial to develop novel diagnostic approaches for determining if peripheral lung nodules are malignant, as such nodules are frequently detected due to the increased use of chest computed tomography scans. To this end, we evaluated levels of napsin A in epithelial lining fluid (ELF), since napsin A has been reported to be an immunohistochemical biomarker for histological diagnosis of primary lung adenocarcinoma.
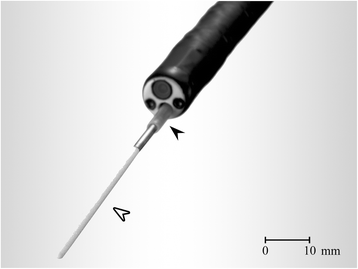
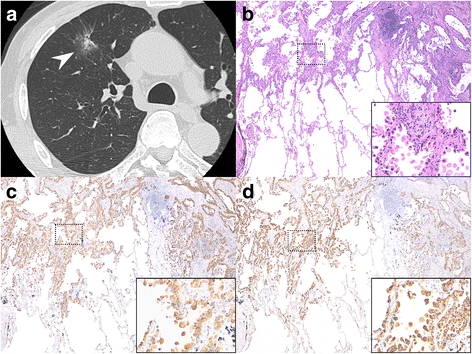
Methods: In consecutive patients with indeterminate peripheral lung nodules, ELF samples were obtained using a bronchoscopic microsampling (BMS) technique. The levels of napsin A and carcinoembryonic antigen (CEA) in ELF at the nodule site were compared with those at the contralateral site. A final diagnosis of primary lung adenocarcinoma was established by surgical resection.
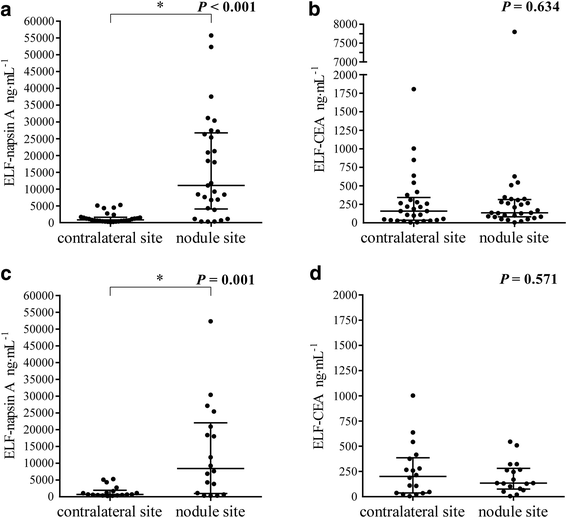
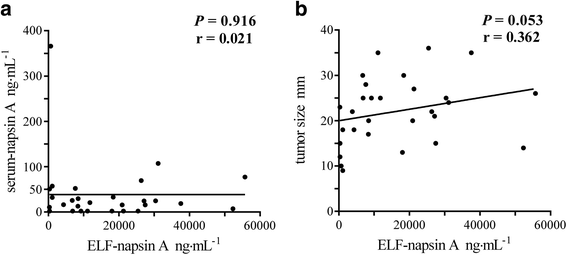
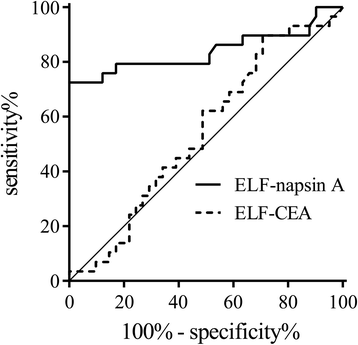
Results: We performed BMS in 43 consecutive patients. Among patients with primary lung adenocarcinoma, the napsin A levels in ELF at the nodule site were markedly higher than those at the contralateral site, while there were no significant differences in CEA levels. Furthermore, in 18 patients who were undiagnosed by bronchoscopy and finally diagnosed by surgery, the napsin A levels in ELF at the nodule site were identically significantly higher than those at the contralateral site. In patients with non-adenocarcinoma, there were no differences in napsin A levels in ELF. The area under the receiver operator characteristic curve for identifying primary lung adenocarcinoma was 0.840 for napsin A and 0.542 for CEA.
Conclusion: Evaluation of napsin A levels in ELF may be useful for distinguishing primary lung adenocarcinoma.
Keywords: Biomarkers; Bronchoscopy; Epithelial lining fluid; Lung cancer diagnosis; Primary lung adenocarcinoma.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the institutional review board of Kagoshima University Medical and Dental Hospital and the committee’s reference number was 24-71. Written informed consent for this study was obtained before bronchoscopy.
Consent for publication
Witten informed consent for publication of all images in this manuscript was obtained from the patients.
Competing interests
Hiromasa Inoue reports grants from Astellas, AstraZeneca, Boehringer-Ingelheim, ChugaiPharm, GlaxoSmithKline, Pfizer, MerckSharp&Dohme, Teijin-Pharma, Torii, personal fees from Astellas, AstraZeneca, Boehringer-Ingelheim, Chugai-Pharm, GlaxoSmithKline, Kyorin, MerckSharp&Dohme, MeijiSeikaPharma, Novartis, Otsuka, Pfizer, outside the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- World Health Organization. Cancer. Fact Sheet Number 297. www.who.int/mediacentre/factsheets/fs297/en/ Last updated February 2015. Accessed 10 Feb 2015.
-
- Kennedy GT, Okusanya OT, Keating JJ, Heitjan DF, Deshpande C, Litzky LA, Albelda SM, Drebin JA, Nie S, Low PS, et al. The optical biopsy: a novel technique for rapid Intraoperative diagnosis of primary pulmonary Adenocarcinomas. Ann Surg. 2015;262(4):602–609. doi: 10.1097/SLA.0000000000001452. - DOI - PMC - PubMed
-
- Slatore CG, Horeweg N, Jett JR, Midthun DE, Powell CA, Wiener RS, Wisnivesky JP, Gould MK. An official American Thoracic Society research statement: a research framework for pulmonary nodule evaluation and management. Am J Respir Crit Care Med. 2015;192(4):500–514. doi: 10.1164/rccm.201506-1082ST. - DOI - PMC - PubMed
-
- Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, Tsubota N. Prognostic significance of perioperative serum carcinoembryonic antigen in non-small cell lung cancer: analysis of 1,000 consecutive resections for clinical stage I disease. Ann Thorac Surg. 2004;78(1):216–221. doi: 10.1016/j.athoracsur.2004.02.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

