Suppression of PTPN6 exacerbates aluminum oxide nanoparticle-induced COPD-like lesions in mice through activation of STAT pathway
- PMID: 29233151
- PMCID: PMC5728016
- DOI: 10.1186/s12989-017-0234-0
Suppression of PTPN6 exacerbates aluminum oxide nanoparticle-induced COPD-like lesions in mice through activation of STAT pathway
Abstract
Background: Inhaled nanoparticles can deposit in the deep lung where they interact with pulmonary cells. Despite numerous studies on pulmonary nanotoxicity, detailed molecular mechanisms of specific nanomaterial-induced lung injury have yet to be identified.
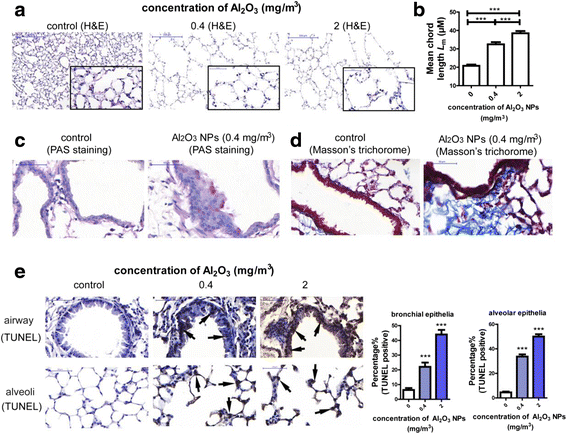
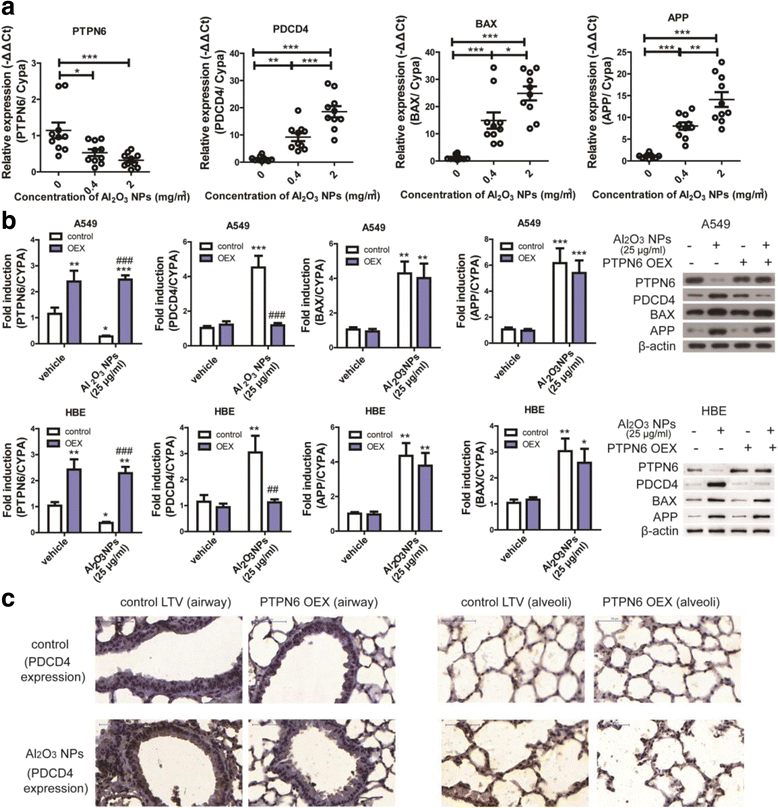
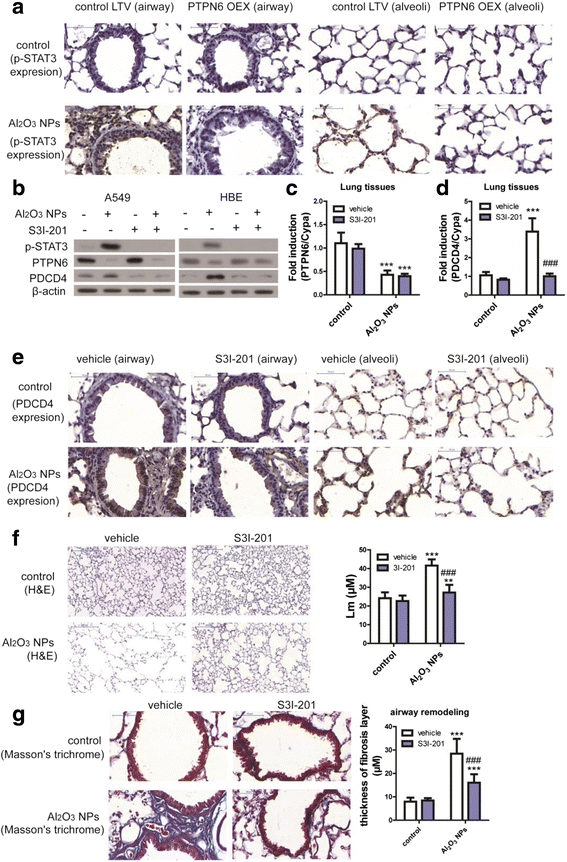
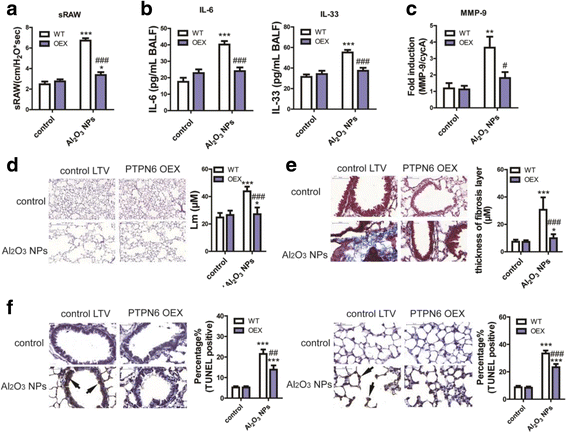
Results: Using whole-body dynamic inhalation model, we studied the interactions between aluminum oxide nanoparticles (Al2O3 NPs) and the pulmonary system in vivo. We found that seven-day-exposure to Al2O3 NPs resulted in emphysema and small airway remodeling in murine lungs, accompanied by enhanced inflammation and apoptosis. Al2O3 NPs exposure led to suppression of PTPN6 and phosphorylation of STAT3, culminating in increased expression of the apoptotic marker PDCD4. Rescue of PTPN6 expression or application of a STAT3 inhibitor, effectively protected murine lungs from inflammation and apoptosis, as well as, in part, from the induction of chronic obstructive pulmonary disease (COPD)-like effects.
Conclusion: In summary, our studies show that inhibition of PTPN6 plays a critical role in Al2O3 NPs-induced COPD-like lesions.
Keywords: Aluminum oxide nanoparticles; PTPN6; Experimental COPD; Inflammation.
Conflict of interest statement
Ethics approval and consent to participate
Animals were treated humanely and all experimental protocols were approved by Committee on Animal Use and Care of Southeast University, China. All the methods in the present study were performed according to approved guidelines.
Consent for publication
Individual person data was not applicable in our manuscript.
Competing interests
The authors declare no competing financial interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Braakhuis HM, Cassee FR, Fokkens PH, de la Fonteyne LJ, Oomen AG, Krystek P, de Jong WH, van Loveren H, Park MV. Identification of the appropriate dose metric for pulmonary inflammation of silver nanoparticles in an inhalation toxicity study. Nanotoxicology. 2016;10:63–73. doi: 10.3109/17435390.2015.1127443. - DOI - PubMed
-
- Sager TM, Wolfarth M, Leonard SS, Morris AM, Porter DW, Castranova V, Holian A. Role of engineered metal oxide nanoparticle agglomeration in reactive oxygen species generation and cathepsin B release in NLRP3 inflammasome activation and pulmonary toxicity. Inhal Toxicol. 2016;28:686–697. doi: 10.1080/08958378.2016.1257664. - DOI - PMC - PubMed
-
- Willhite CC, Karyakina NA, Yokel RA, Yenugadhati N, Wisniewski TM, Arnold IM, Momoli F, Krewski D. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol. 2014;44(Suppl 4):1–80. doi: 10.3109/10408444.2014.934439. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

