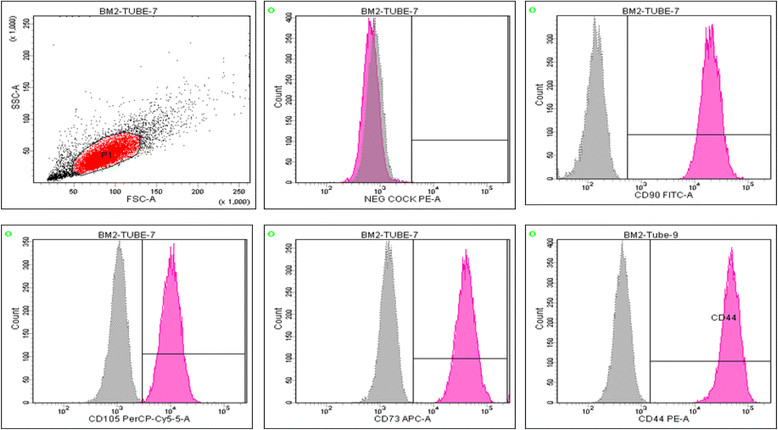
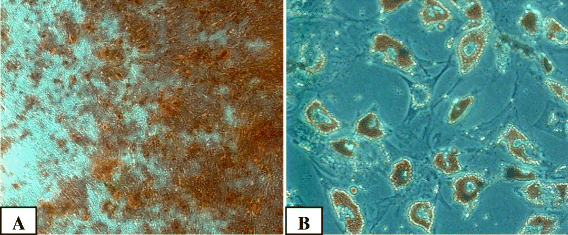
Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study
- PMID: 29233163
- PMCID: PMC5727956
- DOI: 10.1186/s13018-017-0689-6
Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study
Abstract
Background: Knee osteoarthritis (KOA) is a major health problem especially in the aging population. There is a need for safe treatment that restores the cartilage and reduces the symptoms. The use of stem cells is emerging as a possible option for the moderate and severe cases. This study aimed at testing the safety of autologous bone marrow mesenchymal stem cells (BM-MSCs) expanded in vitro when given intra-articularly to patients with stage II and III KOA. As a secondary end point, the study tested the ability of these cells to relieve symptoms and restore the knee cartilage in these patients as judged by normalized knee injury and Osteoarthritis Outcome Score (KOOS) and by magnetic resonance imaging (MRI).
Methods: Thirteen patients with a mean age of 50 years suffering from KOA stages II and III were given two doses of BM-MSCs 1 month apart totaling 61 × 106 ± 0.6 × 106 by intra-articular injection in a phase I prospective clinical trial. Each patient was followed for a minimum of 24 months for any adverse events and for clinical outcome using normalized KOOS. Cartilage thickness was assessed by quantitative MRI T2 at 12 months of follow-up.
Results: No severe adverse events were reported up to 24 months follow-up. Normalized KOOS improved significantly. Mean knee cartilage thickness measured by MRI improved significantly.
Conclusion: BM-MSCs given intra-articularly are safe in knee osteoarthrosis. Despite the limited number of patients in this study, the procedure described significantly improved the KOOS and knee cartilage thickness, indicating that they may enhance the functional outcome as well as the structural component.
Trial registration: ClinicalTrials.gov, NCT02118519.
Keywords: Bone marrow; Intra-articular injection; KOOS score; Knee osteoarthritis; Mesenchymal stem cells.
Conflict of interest statement
Ethics approval and consent to participate
This research was approved by the institutional review board at Jordan University Hospital. All patients were fully informed about the aim and procedures of this work. They were asked to sign a consent formulated in accordance with the Helsinki Declaration. The informed consent included statements authorizing publication of the results.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II).J Transl Med. 2016 Aug 26;14(1):246. doi: 10.1186/s12967-016-0998-2. J Transl Med. 2016. PMID: 27565858 Free PMC article. Clinical Trial.
-
Final results of a phase I-II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration.Knee. 2016 Aug;23(4):647-54. doi: 10.1016/j.knee.2015.08.013. Epub 2016 Jan 11. Knee. 2016. PMID: 26783191 Clinical Trial.
-
Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study.Am J Sports Med. 2017 Oct;45(12):2774-2783. doi: 10.1177/0363546517716641. Epub 2017 Jul 26. Am J Sports Med. 2017. PMID: 28746812
-
Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis.Knee Surg Sports Traumatol Arthrosc. 2020 Dec;28(12):3827-3842. doi: 10.1007/s00167-020-05859-z. Epub 2020 Jan 31. Knee Surg Sports Traumatol Arthrosc. 2020. PMID: 32006075 Free PMC article. Review.
-
Role of Scaffolds, Subchondral, Intra-Articular Injections of Fresh Autologous Bone Marrow Concentrate Regenerative Cells in Treating Human Knee Cartilage Lesions: Different Approaches and Different Results.Int J Mol Sci. 2021 Apr 8;22(8):3844. doi: 10.3390/ijms22083844. Int J Mol Sci. 2021. PMID: 33917689 Free PMC article. Review.
Cited by
-
Mesenchymal Stromal Cell-Based Therapy-An Alternative to Arthroplasty for the Treatment of Osteoarthritis? A State of the Art Review of Clinical Trials.J Clin Med. 2020 Jun 30;9(7):2062. doi: 10.3390/jcm9072062. J Clin Med. 2020. PMID: 32630066 Free PMC article. Review.
-
Modification of mesenchymal stem cells for cartilage-targeted therapy.J Transl Med. 2022 Nov 8;20(1):515. doi: 10.1186/s12967-022-03726-8. J Transl Med. 2022. PMID: 36348497 Free PMC article. Review.
-
Development and Prospect of Intra-Articular Injection in the Treatment of Osteoarthritis: A Review.J Pain Res. 2020 Aug 4;13:1941-1955. doi: 10.2147/JPR.S260878. eCollection 2020. J Pain Res. 2020. PMID: 32801850 Free PMC article. Review.
-
Assessment of the effectiveness and satisfaction of platelet-rich plasma compared with hyaluronic acid in knee osteoarthritis at minimum 7-year follow-up: A post hoc analysis of a randomized controlled trial.Front Bioeng Biotechnol. 2022 Nov 25;10:1062371. doi: 10.3389/fbioe.2022.1062371. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36507262 Free PMC article.
-
Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis.J Transl Med. 2020 Sep 18;18(1):356. doi: 10.1186/s12967-020-02530-6. J Transl Med. 2020. PMID: 32948200 Free PMC article. Clinical Trial.
References
-
- Dillon CF, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33(11):2271–2279. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials