Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo
- PMID: 29233174
- PMCID: PMC5727954
- DOI: 10.1186/s12967-017-1356-8
Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo
Abstract
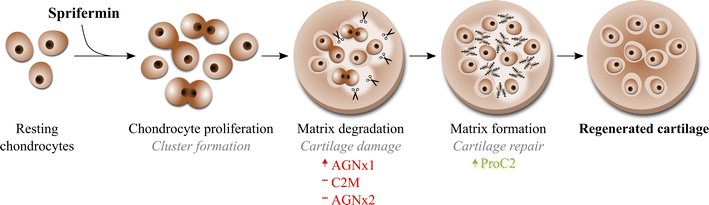
Background: Sprifermin (recombinant human fibroblast growth factor 18) is in clinical development as a potential disease-modifying osteoarthritis drug (DMOAD). In vitro studies have shown that cartilage regenerative properties of sprifermin involve chondrocyte proliferation and extracellular matrix (ECM) production. To gain further insight into the process of sprifermin in the cartilage tissue, this study aimed at investigating the ECM turnover of articular cartilage explants in a longitudinal manner.
Methods: Bovine full-depth articular cartilage explants were stimulated with sprifermin or placebo at weekly intervals, similar to the dosing regimen used in clinical trials. Pre-culturing with oncostatin M and tumour necrosis factor-α, was also used to induce an inflammatory state before treatment. Metabolic activity was measured using AlamarBlue, and chondrocyte proliferation was visualized by immuno-histochemical detection of proliferating cell nuclear antigen. ECM turnover was quantified by biomarker ELISAs; ProC2 reflecting type II collagen formation, CS846 reflecting aggrecan formation, active MMP9, C2M and AGNx2 reflecting matrix metalloproteinase activity, and AGNx1 reflecting aggrecanase activity.
Results: Sprifermin was able to reach the chondrocytes through the extracellular matrix, as it increased cell proliferation and metabolic activity of explants. ProC2 and CS846 was dose-dependently increased (P < 0.05) by sprifermin compared to placebo, while C2M and AGNx2 were unaffected, active MMP9 was slightly decreased, and AGNx1 was slightly increased. Over the course of treatment, the temporal order of ECM turnover responses was AGNx1, then ProC2, followed by CS846 and MMP9. Pro-inflammatory activation of the explants diminished the ECM turnover responses otherwise observed under non-inflammatory conditions.
Conclusions: The data suggest that sprifermin has chondrogenic effects on articular cartilage ex vivo, exerted through a sequential process of ECM turnover; aggrecan degradation seems to occur first, while type II collagen and aggrecan production increased at a later time point. In addition, it was observed that these chondrogenic effects are dependent on the inflammatory status of the cartilage prior to treatment.
Keywords: Chondrocyte and cartilage biology; Osteoarthritis; Other therapeutics.
Figures



Similar articles
-
Sprifermin (rhFGF18) versus vehicle induces a biphasic process of extracellular matrix remodeling in human knee OA articular cartilage ex vivo.Sci Rep. 2020 Apr 7;10(1):6011. doi: 10.1038/s41598-020-63216-z. Sci Rep. 2020. PMID: 32265494 Free PMC article.
-
Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix.Osteoarthritis Cartilage. 2017 Nov;25(11):1858-1867. doi: 10.1016/j.joca.2017.08.004. Epub 2017 Aug 18. Osteoarthritis Cartilage. 2017. PMID: 28823647
-
Recombinant human FGF18 preserves depth-dependent mechanical inhomogeneity in articular cartilage.Eur Cell Mater. 2019 Aug 8;38:23-34. doi: 10.22203/eCM.v038a03. Eur Cell Mater. 2019. PMID: 31393594 Free PMC article.
-
Sprifermin: a recombinant human fibroblast growth factor 18 for the treatment of knee osteoarthritis.Expert Opin Investig Drugs. 2021 Sep;30(9):923-930. doi: 10.1080/13543784.2021.1972970. Epub 2021 Sep 3. Expert Opin Investig Drugs. 2021. PMID: 34427483 Review.
-
Mechanical Cues: Bidirectional Reciprocity in the Extracellular Matrix Drives Mechano-Signalling in Articular Cartilage.Int J Mol Sci. 2021 Dec 18;22(24):13595. doi: 10.3390/ijms222413595. Int J Mol Sci. 2021. PMID: 34948394 Free PMC article. Review.
Cited by
-
Evaluation of serum ARGS neoepitope as an osteoarthritis biomarker using a standardized model for exercise-induced cartilage extra cellular matrix turnover.Osteoarthr Cartil Open. 2020 Apr 3;2(2):100060. doi: 10.1016/j.ocarto.2020.100060. eCollection 2020 Jun. Osteoarthr Cartil Open. 2020. PMID: 36474584 Free PMC article.
-
Soluble biological markers in osteoarthritis.Ther Adv Musculoskelet Dis. 2021 Sep 29;13:1759720X211040300. doi: 10.1177/1759720X211040300. eCollection 2021. Ther Adv Musculoskelet Dis. 2021. PMID: 34616494 Free PMC article. Review.
-
Targeted Therapy of Osteoarthritis via Intra-Articular Delivery of Lipid-Nanoparticle-Encapsulated Recombinant Human FGF18 mRNA.Adv Healthc Mater. 2024 Nov;13(29):e2400804. doi: 10.1002/adhm.202400804. Epub 2024 Oct 4. Adv Healthc Mater. 2024. PMID: 39363784 Free PMC article.
-
Recombinant fibroblast growth factor-18 (sprifermin) enhances microfracture-induced cartilage healing.J Orthop Res. 2022 Mar;40(3):553-564. doi: 10.1002/jor.25063. Epub 2021 May 12. J Orthop Res. 2022. PMID: 33934397 Free PMC article.
-
Cartilage tissue turnover increases with high- compared to low-intensity resistance training in patients with knee OA.Arthritis Res Ther. 2023 Feb 10;25(1):22. doi: 10.1186/s13075-023-03000-2. Arthritis Res Ther. 2023. PMID: 36765372 Free PMC article. Clinical Trial.
References
-
- Mori Y, Saito T, Chang SH, Kobayashi H, Ladel CH, Guehring H, et al. Identification of fibroblast growth factor-18 as a molecule to protect adult articular cartilage by gene expression profiling. J Biol Chem. 2014;289:10192–200. http://www.ncbi.nlm.nih.gov/pubmed/24577103. Accessed 6 Mar 2014. - PMC - PubMed
-
- Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr. Cartil. 2005;13:623–31. http://www.ncbi.nlm.nih.gov/pubmed/15896984. Accessed 11 July 2013. - PubMed
-
- Dahlberg LE, Aydemir A, Muurahainen N, Gühring H, Fredberg Edebo H, Krarup-Jensen N, et al. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin Exp Rheumatol. 2016;34:445–50. http://www.ncbi.nlm.nih.gov/pubmed/27050139. - PubMed
-
- Lohmander LS, Hellot S, Dreher D, Krantz EFW, Kruger DS, Guermazi A, et al. Intra-articular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014;66:1820–31. http://www.ncbi.nlm.nih.gov/pubmed/24740822. Accessed 25 Apr 2014. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

