Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo
- PMID: 29233174
- PMCID: PMC5727954
- DOI: 10.1186/s12967-017-1356-8
Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo
Abstract
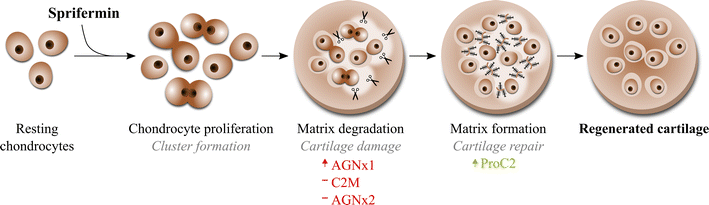
Background: Sprifermin (recombinant human fibroblast growth factor 18) is in clinical development as a potential disease-modifying osteoarthritis drug (DMOAD). In vitro studies have shown that cartilage regenerative properties of sprifermin involve chondrocyte proliferation and extracellular matrix (ECM) production. To gain further insight into the process of sprifermin in the cartilage tissue, this study aimed at investigating the ECM turnover of articular cartilage explants in a longitudinal manner.
Methods: Bovine full-depth articular cartilage explants were stimulated with sprifermin or placebo at weekly intervals, similar to the dosing regimen used in clinical trials. Pre-culturing with oncostatin M and tumour necrosis factor-α, was also used to induce an inflammatory state before treatment. Metabolic activity was measured using AlamarBlue, and chondrocyte proliferation was visualized by immuno-histochemical detection of proliferating cell nuclear antigen. ECM turnover was quantified by biomarker ELISAs; ProC2 reflecting type II collagen formation, CS846 reflecting aggrecan formation, active MMP9, C2M and AGNx2 reflecting matrix metalloproteinase activity, and AGNx1 reflecting aggrecanase activity.
Results: Sprifermin was able to reach the chondrocytes through the extracellular matrix, as it increased cell proliferation and metabolic activity of explants. ProC2 and CS846 was dose-dependently increased (P < 0.05) by sprifermin compared to placebo, while C2M and AGNx2 were unaffected, active MMP9 was slightly decreased, and AGNx1 was slightly increased. Over the course of treatment, the temporal order of ECM turnover responses was AGNx1, then ProC2, followed by CS846 and MMP9. Pro-inflammatory activation of the explants diminished the ECM turnover responses otherwise observed under non-inflammatory conditions.
Conclusions: The data suggest that sprifermin has chondrogenic effects on articular cartilage ex vivo, exerted through a sequential process of ECM turnover; aggrecan degradation seems to occur first, while type II collagen and aggrecan production increased at a later time point. In addition, it was observed that these chondrogenic effects are dependent on the inflammatory status of the cartilage prior to treatment.
Keywords: Chondrocyte and cartilage biology; Osteoarthritis; Other therapeutics.
Figures



References
-
- Mori Y, Saito T, Chang SH, Kobayashi H, Ladel CH, Guehring H, et al. Identification of fibroblast growth factor-18 as a molecule to protect adult articular cartilage by gene expression profiling. J Biol Chem. 2014;289:10192–200. http://www.ncbi.nlm.nih.gov/pubmed/24577103. Accessed 6 Mar 2014. - PMC - PubMed
-
- Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr. Cartil. 2005;13:623–31. http://www.ncbi.nlm.nih.gov/pubmed/15896984. Accessed 11 July 2013. - PubMed
-
- Dahlberg LE, Aydemir A, Muurahainen N, Gühring H, Fredberg Edebo H, Krarup-Jensen N, et al. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin Exp Rheumatol. 2016;34:445–50. http://www.ncbi.nlm.nih.gov/pubmed/27050139. - PubMed
-
- Lohmander LS, Hellot S, Dreher D, Krantz EFW, Kruger DS, Guermazi A, et al. Intra-articular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014;66:1820–31. http://www.ncbi.nlm.nih.gov/pubmed/24740822. Accessed 25 Apr 2014. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

