Immature morphological properties in subcellular-scale structures in the dentate gyrus of Schnurri-2 knockout mice: a model for schizophrenia and intellectual disability
- PMID: 29233179
- PMCID: PMC5727961
- DOI: 10.1186/s13041-017-0339-2
Immature morphological properties in subcellular-scale structures in the dentate gyrus of Schnurri-2 knockout mice: a model for schizophrenia and intellectual disability
Abstract
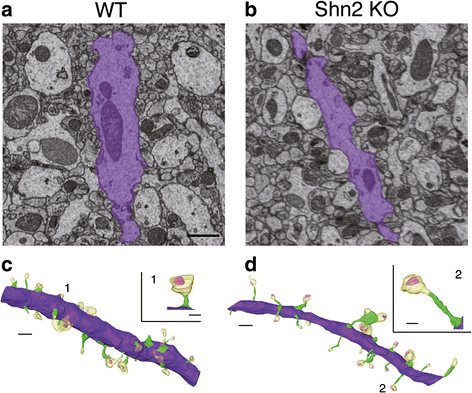
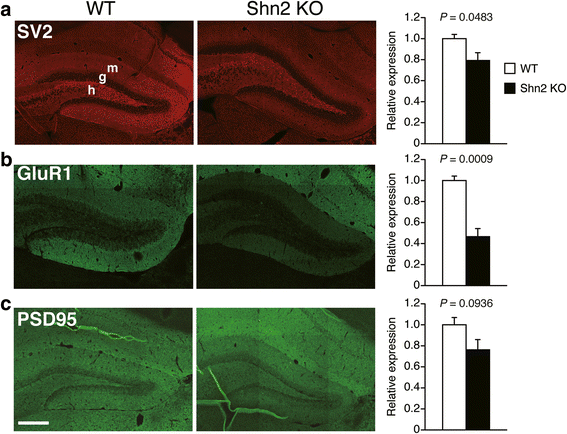
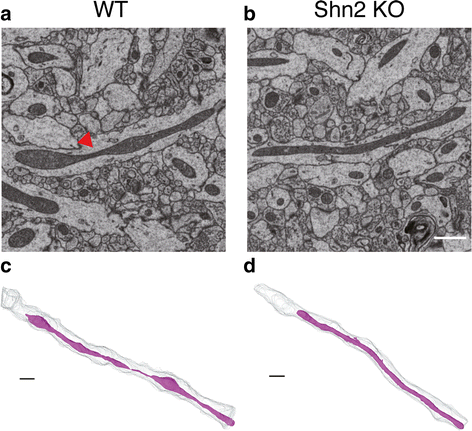
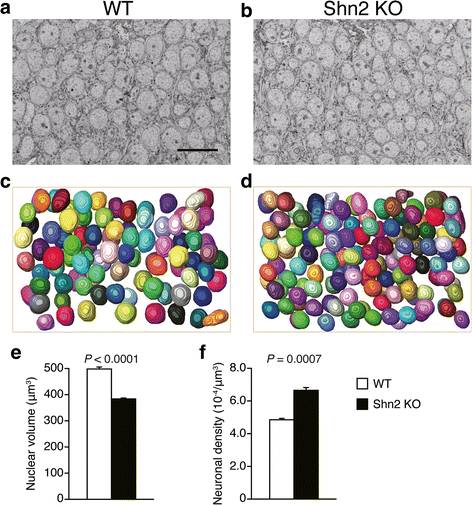
Accumulating evidence suggests that subcellular-scale structures such as dendritic spine and mitochondria may be involved in the pathogenesis/pathophysiology of schizophrenia and intellectual disability. Previously, we proposed mice lacking Schnurri-2 (Shn2; also called major histocompatibility complex [MHC]-binding protein 2 [MBP-2], or human immunodeficiency virus type I enhancer binding protein 2 [HIVEP2]) as a schizophrenia and intellectual disability model with mild chronic inflammation. In the mutants' brains, there are increases in C4b and C1q genes, which are considered to mediate synapse elimination during postnatal development. However, morphological properties of subcellular-scale structures such as dendritic spine in Shn2 knockout (KO) mice remain unknown. In this study, we conducted three-dimensional morphological analyses in subcellular-scale structures in dentate gyrus granule cells of Shn2 KO mice by serial block-face scanning electron microscopy. Shn2 KO mice showed immature dendritic spine morphology characterized by increases in spine length and decreases in spine diameter. There was a non-significant tendency toward decrease in spine density of Shn2 KO mice over wild-type mice, and spine volume was indistinguishable between genotypes. Shn2 KO mice exhibited a significant reduction in GluR1 expression and a nominally significant decrease in SV2 expression, while PSD95 expression had a non-significant tendency to decrease in Shn2 KO mice. There were significant decreases in dendrite diameter, nuclear volume, and the number of constricted mitochondria in the mutants. Additionally, neuronal density was elevated in Shn2 KO mice. These results suggest that Shn2 KO mice serve as a unique tool for investigating morphological abnormalities of subcellular-scale structures in schizophrenia, intellectual disability, and its related disorders.
Keywords: 3D electron microscopy; Intellectual disability; Mouse model; Schizophrenia.
Conflict of interest statement
Ethics approval
All procedures were approved by the Institutional Animal Care and Use Committee of Fujita Health University.
Consent for publication
Not applicable.
Competing interests
TM received research grants from Astellas Pharma Inc. and Toyama Chemical Co., Ltd. Other authors have no conflict of interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

